Carboxylic acids and esters
Carboxylic acid is also known as "organic acid". It refers to the general term of the organic compound formed through the combination between hydrocarbon group and carboxyl group with the general formula being RCOOH.It is mostly in the form of an ester to be widespread in nature. Based on the hydrocarbon structure, it is divided into: 1 aliphatic carboxylic acid: it is formed through connection between aliphatic hydrocarbon group and carboxyl group, such as acetic acid CH3COOH. 2 aromatic carboxylic acids: it is formed through the connection between aromatic rings with a carboxyl group, such as benzoic acid C6H5-COOH. 3 cyclic acid; it is formed through the connection between carboxyl group and cyclic hydrocarbon group such as cyclohexane carboxylic acid. 4 saturated carboxylic acids: it refers to saturated hydrocarbon-containing acids, such as butyric acid CH3CH2CH2COOH. 5 unsaturated carboxylic: acids; it refers to acid containing an unsaturated hydrocarbon group such as acrylic acid CH2 = CHCOOH.
Based on the number of carboxyl groups contained in the molecules, it can be divided into monobasic acid, dibasic acids and poly-acids. Those which have substituents groups contained inside the hydrocarbon are called as "substituted carboxylic acid." Saturated mono-carboxylic acids containing one to nine carbon atoms inside the molecule appear as liquid while those containing more than ten carbon atoms appear as solid while aromatic carboxylic acids and saturated dicarboxylic acids both appear as solid. Carboxylic acids have weak acidicity, and have been largely applied to the manufacturing of esters, acyl chlorides, amides and anhydrides, with reduction of the carboxylic acid yielding the corresponding alcohol. A large number of carboxylic acids have widely applied to dyes, rubber, pharmaceuticals, perfumes, cosmetics, light industry and other industries.
Esters spices refer to the ester compound synthesized through the reaction between alcohols and carboxylic acids. It is a very important large class in spice. Depending on the raw materials used in the synthesis, it can be roughly divided into: aliphatic carboxylates, aromatic carboxylate ester, alkene & alkyne unsaturated bond-containing carboxylic acid ester, and other esters.
The main features of ester series spices are: carboxylic ester can be taken as the product of the substitution of the hydroxy group in acid with alkoxy group. Because the hydroxyl group contained in a carboxyl group may be substituted with one or more alkoxy groups, thus leading to the synthesis of monoester, diester or polyesters. Furthermore, there may also be several hydroxy groups contained in the alcohols, and therefore its alkoxy groups can be connected with one or more carboxyl groups to yield mono- or carboxylate. The aroma, fragrance type, fragrance intensity and features are all related to the structure of the esters. The esters formed through low-grade carboxylic acid and low grade alcohol generally appear as volatile liquid with aroma of flowers, fruit and grass. The ester formed between low grade carboxylic acid and low grade terpene has flower aroma. Esters containing aromatic groups mostly have flower aroma. Esters results from aromatic acids and aromatic alcohols, although with not very strong aroma, has relative higher boiling points and long-lasting aroma. Esters spices are widely distributed in nature with existence in plant roots, stems, leaves, fruits, seeds, bark, flowers and other parts as well as in the secretion products of some animals. There are even more kinds of artificially synthetic ester spices. Because of the easy process of synthesis of esters and the wide source of raw materials, there are many fragrance types for the synthetic esters; the price is also low, and therefore they have achieved widely applications.
Fatty acid esters spices occupy an important status in the fragrance industry, which is characterized by variety, easy synthesis and low prices, and it has been widely used in daily flavor, food flavor as well as industrial flavor. The mainly preparation methods include the esterification reaction between alcohol and fatty acid, the reaction between alcohol and acid anhydride, the reaction between acyl chloride and alcohol, interchange reaction of ester and the reaction between carboxylic acid salt and alkyl halide.
The aromatic esters spices mainly include benzoate, phenylacetic acid ester, cinnamic acid ester and salicylic acid esters. Methods of synthesizing mainly include esterification between alcohol and aromatic acids, reaction between alcohol and benzoyl chloride, ester exchange reaction as well as the reaction between alkyl halide and aromatic acid salt. They play an important role in the daily flavors and edible flavors.
- Structure:
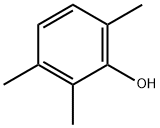
- Chemical Name:2,3,6-Trimethylphenol
- CAS:2416-94-6
- MF:C9H12O
- Structure:
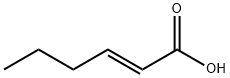
- Chemical Name:trans-2-Hexenoic acid
- CAS:13419-69-7
- MF:C6H10O2
- Structure:
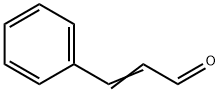
- Chemical Name:Cinnamaldehyde
- CAS:104-55-2
- MF:C9H8O
- Structure:
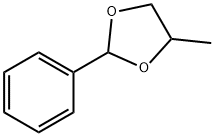
- Chemical Name:Benzaldehyde propylene glycol acetal
- CAS:2568-25-4
- MF:C10H12O2
- Structure:
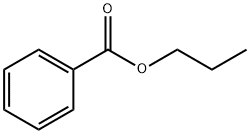
- Chemical Name:PROPYL BENZOATE
- CAS:2315-68-6
- MF:C10H12O2
- Structure:
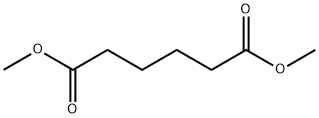
- Chemical Name:Dimethyl adipate
- CAS:627-93-0
- MF:C8H14O4
- Structure:
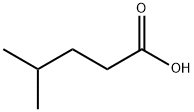
- Chemical Name:4-Methylvaleric acid
- CAS:646-07-1
- MF:C6H12O2
- Structure:
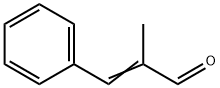
- Chemical Name:α-Methylcinnamaldehyde
- CAS:101-39-3
- MF:C10H10O
- Structure:
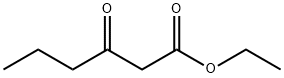
- Chemical Name:Ethyl butyrylacetate
- CAS:3249-68-1
- MF:C8H14O3
- Structure:
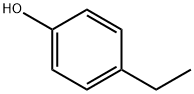
- Chemical Name:4-Ethylphenol
- CAS:123-07-9
- MF:C8H10O
- Structure:
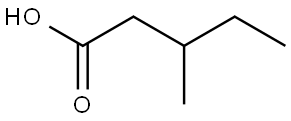
- Chemical Name:DL-3-Methylvaleric acid
- CAS:105-43-1
- MF:C6H12O2
- Structure:
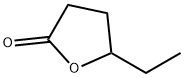
- Chemical Name:4-Hexanolide
- CAS:695-06-7
- MF:C6H10O2
- Structure:
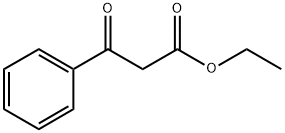
- Chemical Name:Ethyl benzoylacetate
- CAS:94-02-0
- MF:C11H12O3
- Structure:
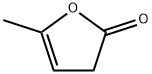
- Chemical Name:alpha-Angelica lactone
- CAS:591-12-8
- MF:C5H6O2
- Structure:
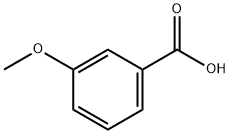
- Chemical Name:3-Methoxybenzoic acid
- CAS:586-38-9
- MF:C8H8O3
- Structure:
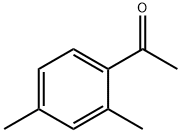
- Chemical Name:2',4'-Dimethylacetophenone
- CAS:89-74-7
- MF:C10H12O
- Structure:
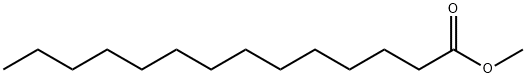
- Chemical Name:METHYL MYRISTATE
- CAS:124-10-7
- MF:C15H30O2
- Structure:
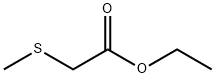
- Chemical Name:ETHYL (METHYLTHIO)ACETATE
- CAS:4455-13-4
- MF:C5H10O2S
- Structure:
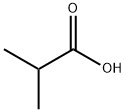
- Chemical Name:Isobutyric acid
- CAS:79-31-2
- MF:C4H8O2
- Structure:
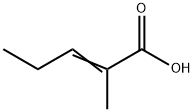
- Chemical Name:2-Methyl-2-pentenoic acid
- CAS:3142-72-1
- MF:C6H10O2
- Structure:
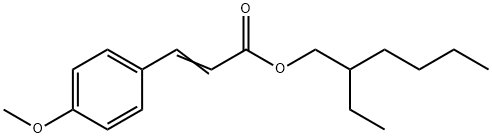
- Chemical Name:Octyl 4-methoxycinnamate
- CAS:5466-77-3
- MF:C18H26O3
- Structure:
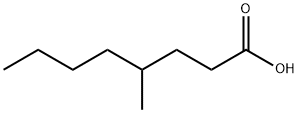
- Chemical Name:4-Methyloctanoic acid
- CAS:54947-74-9
- MF:C9H18O2
- Structure:
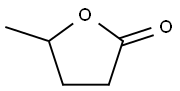
- Chemical Name:γ-Valerolactone
- CAS:108-29-2
- MF:C5H8O2
- Structure:
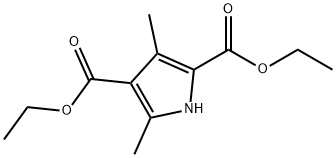
- Chemical Name:Diethyl 2,4-dimethylpyrrole-3,5-dicarboxylate
- CAS:2436-79-5
- MF:C12H17NO4
- Structure:
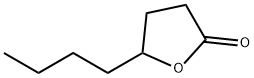
- Chemical Name:gamma-Octanoic lactone
- CAS:104-50-7
- MF:C8H14O2
- Structure:
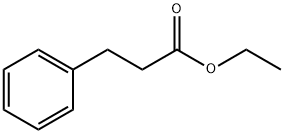
- Chemical Name:Ethyl 3-phenylpropionate
- CAS:2021-28-5
- MF:C11H14O2
- Structure:
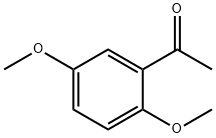
- Chemical Name:2',5'-Dimethoxyacetophenone
- CAS:1201-38-3
- MF:C10H12O3
- Structure:
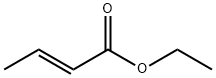
- Chemical Name:Ethyl crotonate
- CAS:623-70-1
- MF:C6H10O2
- Structure:
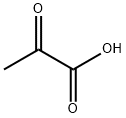
- Chemical Name:Pyruvic acid
- CAS:127-17-3
- MF:C3H4O3
- Structure:
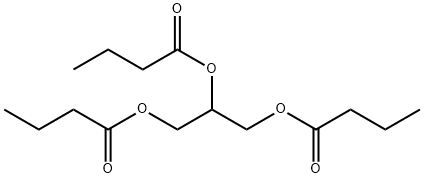
- Chemical Name:Tributyrin
- CAS:60-01-5
- MF:C15H26O6
- Structure:
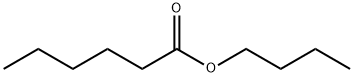
- Chemical Name:Butyl hexanoate
- CAS:626-82-4
- MF:C10H20O2
- Structure:
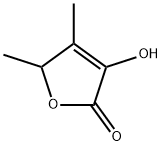
- Chemical Name:4,5-Dimethyl-3-hydroxy-2,5-dihydrofuran-2-one
- CAS:28664-35-9
- MF:C6H8O3
- Structure:
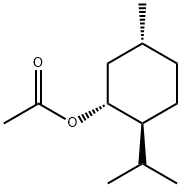
- Chemical Name:L-Menthyl acetate
- CAS:2623-23-6
- MF:C12H22O2
- Structure:
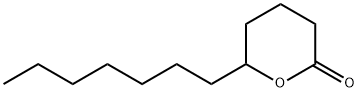
- Chemical Name:delta-Dodecalactone
- CAS:713-95-1
- MF:C12H22O2
- Structure:
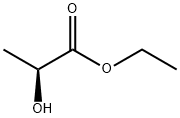
- Chemical Name:Ethyl L(-)-lactate
- CAS:687-47-8
- MF:C5H10O3
- Structure:
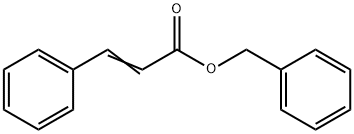
- Chemical Name:Benzyl cinnamate
- CAS:103-41-3
- MF:C16H14O2
- Structure:
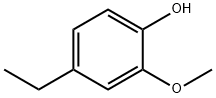
- Chemical Name:4-Ethyl-2-methoxyphenol
- CAS:2785-89-9
- MF:C9H12O2
- Structure:
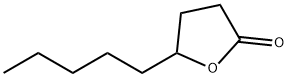
- Chemical Name:gamma-Nonanolactone
- CAS:104-61-0
- MF:C9H16O2
- Structure:

- Chemical Name:Ethyl levulinate
- CAS:539-88-8
- MF:C7H12O3
- Structure:
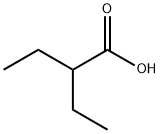
- Chemical Name:2-Ethylbutyric acid
- CAS:88-09-5
- MF:C6H12O2
- Structure:
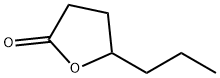
- Chemical Name:4-Heptanolide
- CAS:105-21-5
- MF:C7H12O2
- Structure:
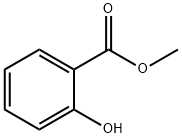
- Chemical Name:Methyl salicylate
- CAS:119-36-8
- MF:C8H8O3
- Structure:
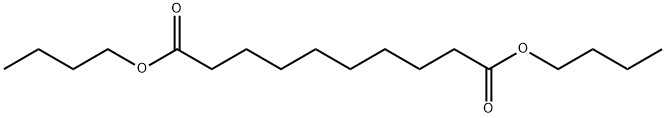
- Chemical Name:Dibutyl sebacate
- CAS:109-43-3
- MF:C18H34O4
- Structure:
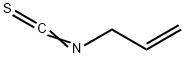
- Chemical Name:Allyl isothiocyanate
- CAS:57-06-7
- MF:C4H5NS
- Structure:

- Chemical Name:Butyl formate
- CAS:592-84-7
- MF:C5H10O2
- Structure:
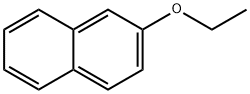
- Chemical Name:2-Ethoxynaphthalene
- CAS:93-18-5
- MF:C12H12O
- Structure:
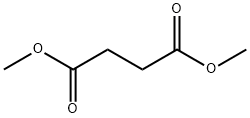
- Chemical Name:Dimethyl succinate
- CAS:106-65-0
- MF:C6H10O4
- Structure:
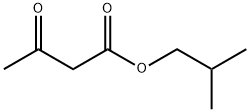
- Chemical Name:Isobutyl acetoacetate
- CAS:7779-75-1
- MF:C8H14O3
- Structure:
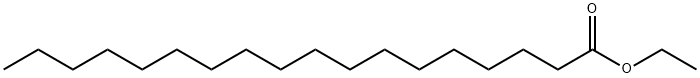
- Chemical Name:ETHYL STEARATE
- CAS:111-61-5
- MF:C20H40O2
- Structure:
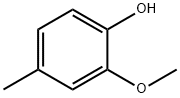
- Chemical Name:2-Methoxy-4-methylphenol
- CAS:93-51-6
- MF:C8H10O2
- Structure:
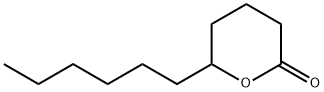
- Chemical Name:Undecanolactone
- CAS:710-04-3
- MF:C11H20O2
- Structure:
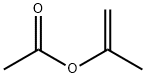
- Chemical Name:Isopropenyl acetate
- CAS:108-22-5
- MF:C5H8O2
- Structure:
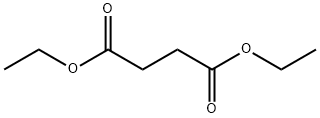
- Chemical Name:Diethyl succinate
- CAS:123-25-1
- MF:C8H14O4
- Structure:
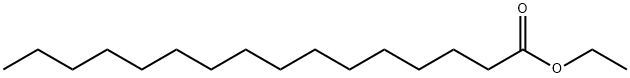
- Chemical Name:Palmitic acid ethyl ester
- CAS:628-97-7
- MF:C18H36O2
- Structure:
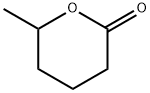
- Chemical Name:delta-Hexalactone
- CAS:823-22-3
- MF:C6H10O2
- Structure:
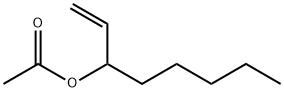
- Chemical Name:1-Octen-3-yl acetate
- CAS:2442-10-6
- MF:C10H18O2
- Structure:
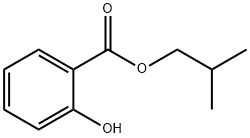
- Chemical Name:Isobutyl salicylate
- CAS:87-19-4
- MF:C11H14O3
- Structure:
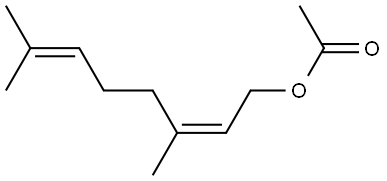
- Chemical Name:NERYL ACETATE
- CAS:141-12-8
- MF:C12H20O2
- Structure:
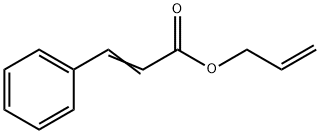
- Chemical Name:ALLYL CINNAMATE
- CAS:1866-31-5
- MF:C12H12O2
- Structure:
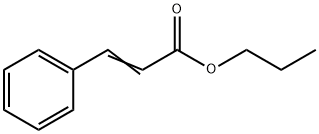
- Chemical Name:Propyl cinnamate
- CAS:7778-83-8
- MF:C12H14O2
- Structure:
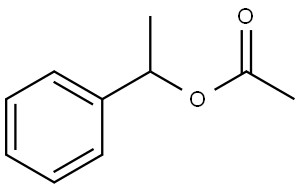
- Chemical Name:Styralyl acetate
- CAS:93-92-5
- MF:C10H12O2
- Structure:
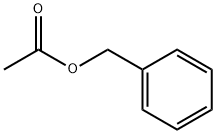
- Chemical Name:Benzyl acetate
- CAS:140-11-4
- MF:C9H10O2
- Structure:
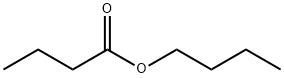
- Chemical Name:Butyl butyrate
- CAS:109-21-7
- MF:C8H16O2
- Structure:
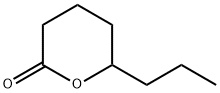
- Chemical Name:5-Hydroxyoctanoic acid lactone
- CAS:698-76-0
- MF:C8H14O2
- Structure:
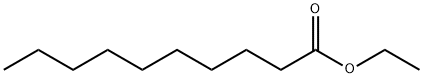
- Chemical Name:Ethyl caprate
- CAS:110-38-3
- MF:C12H24O2
- Structure:
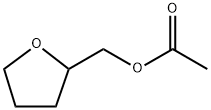
- Chemical Name:TETRAHYDROFURFURYL ACETATE
- CAS:637-64-9
- MF:C7H12O3
- Structure:
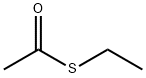
- Chemical Name:Ethanethioic acid S-ethyl ester
- CAS:625-60-5
- MF:C4H8OS
- Structure:
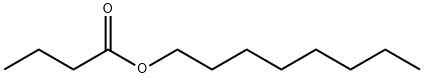
- Chemical Name:OCTYL BUTYRATE
- CAS:110-39-4
- MF:C12H24O2
- Structure:
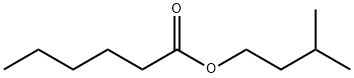
- Chemical Name:ISOAMYL HEXANOATE
- CAS:2198-61-0
- MF:C11H22O2
- Structure:
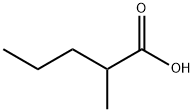
- Chemical Name:2-METHYLVALERIC ACID
- CAS:97-61-0
- MF:C6H12O2
- Structure:
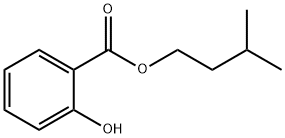
- Chemical Name:Isoamyl o-hydroxybenzoate
- CAS:87-20-7
- MF:C12H16O3
- Structure:
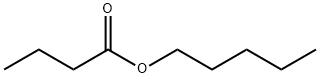
- Chemical Name:AMYL BUTYRATE
- CAS:540-18-1
- MF:C9H18O2
- Structure:
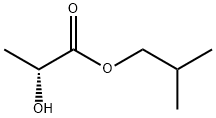
- Chemical Name:(+)-Isobutyl D-lactate
- CAS:61597-96-4
- MF:C7H14O3
- Structure:
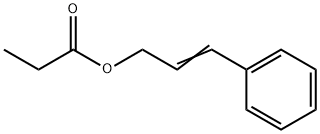
- Chemical Name:Cinnamyl propionate
- CAS:103-56-0
- MF:C12H14O2
- Structure:
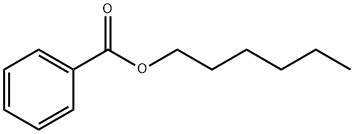
- Chemical Name:Hexyl benzoate
- CAS:6789-88-4
- MF:C13H18O2
- Structure:

- Chemical Name:Diethyl malonate
- CAS:105-53-3
- MF:C7H12O4
- Structure:
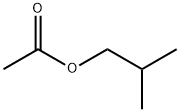
- Chemical Name:Isobutyl acetate
- CAS:110-19-0
- MF:C6H12O2
- Structure:
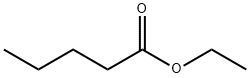
- Chemical Name:Ethyl valerate
- CAS:539-82-2
- MF:C7H14O2
- Structure:
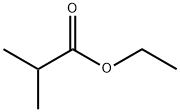
- Chemical Name:Ethyl isobutyrate
- CAS:97-62-1
- MF:C6H12O2
- Structure:
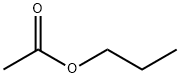
- Chemical Name:Propyl acetate
- CAS:109-60-4
- MF:C5H10O2
- Structure:
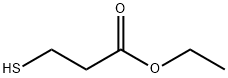
- Chemical Name:Ethyl 3-mercaptopropionate
- CAS:5466-06-8
- MF:C5H10O2S
- Structure:
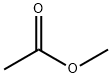
- Chemical Name:Methyl acetate
- CAS:79-20-9
- MF:C3H6O2
- Structure:
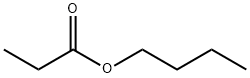
- Chemical Name:Butyl propionate
- CAS:590-01-2
- MF:C7H14O2
- Structure:
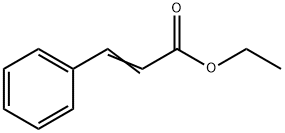
- Chemical Name:Ethyl cinnamate
- CAS:103-36-6
- MF:C11H12O2
- Structure:
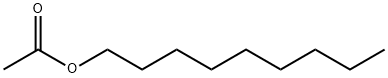
- Chemical Name:Nonyl acetate
- CAS:143-13-5
- MF:C11H22O2
- Structure:
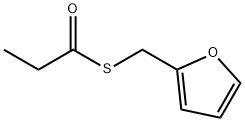
- Chemical Name:Furfuryl thiopropionate
- CAS:59020-85-8
- MF:C8H10O2S
- Structure:
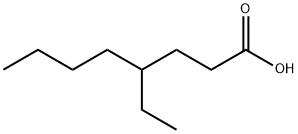
- Chemical Name:4-Ethyloctanoic acid
- CAS:16493-80-4
- MF:C10H20O2
- Structure:
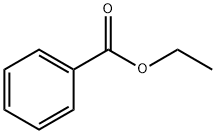
- Chemical Name:Ethyl benzoate
- CAS:93-89-0
- MF:C9H10O2
- Structure:
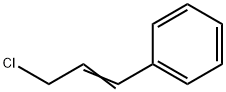
- Chemical Name:Cinnamyl chloride
- CAS:2687-12-9
- MF:C9H9Cl
- Structure:
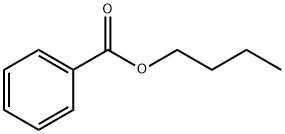
- Chemical Name:Butyl benzoate
- CAS:136-60-7
- MF:C11H14O2
- Structure:
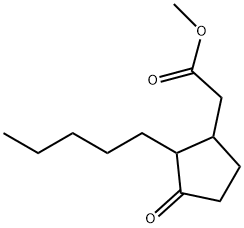
- Chemical Name:Methyl dihydrojasmonate
- CAS:24851-98-7
- MF:C13H22O3
- Structure:
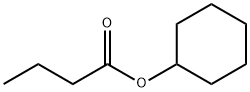
- Chemical Name:CYCLOHEXYL BUTYRATE
- CAS:1551-44-6
- MF:C10H18O2
- Structure:
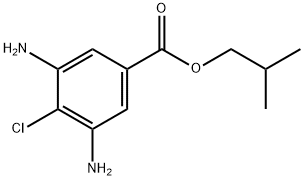
- Chemical Name:Isobutyl 3,5-diamino-4-chloro benzoate
- CAS:32961-44-7
- MF:C11H15ClN2O2
- Structure:
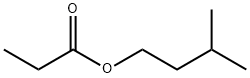
- Chemical Name:Isoamyl propionate
- CAS:105-68-0
- MF:C8H16O2
- Structure:
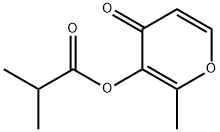
- Chemical Name:Maltol isobutyrate
- CAS:65416-14-0
- MF:C10H12O4
- Structure:
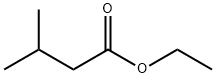
- Chemical Name:Ethyl isovalerate
- CAS:108-64-5
- MF:C7H14O2
- Structure:
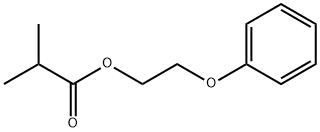
- Chemical Name:Phenoxyethyl isobutyrate
- CAS:103-60-6
- MF:C12H16O3
- Structure:
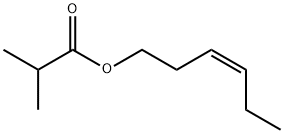
- Chemical Name:CIS-3-HEXENYL ISOBUTYRATE
- CAS:41519-23-7
- MF:C10H18O2
- Structure:
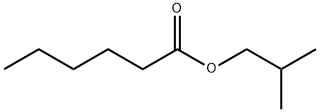
- Chemical Name:Isobutyl hexanoate
- CAS:105-79-3
- MF:C10H20O2
- Structure:
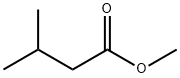
- Chemical Name:Methyl isovalerate
- CAS:556-24-1
- MF:C6H12O2