Oxides and peroxides
Oxide is an inorganic compound consisting of two elements, one of which is oxygen. Except helium, neon, krypton and other rare elements, all of other elements can form an oxide with oxygen. According to their acidity, oxides are generally divided into alkaline oxides, acid oxides and amphoteric oxides. Basic oxide is capable of forming a salt with an acid, acidic oxide is capable of reacting with the alkali solution to produce a salt, and the amphoteric oxide is capable of both above. Some oxides such as carbon monoxide (CO), nitric oxide (NO), etc., are known as neutral oxides for they react neither with an acid nor with a base. Oxides are widely distributed in nature, such carbon dioxide in the air and silicon dioxide (SiO2), the main component of quartz sand. And the principal component of hematite, which is a raw material for ion, is ferric oxide.
The main applications of oxides: ① catalyst, ② inorganic pigments, ③ magnetic material, ④ optical material, ⑤ superconducting material, ⑥ chemical separation.
Peroxide, also known as superoxide, is a class of compounds consisting of peroxide groups (-O-O-). Peroxide can be inorganic or organic . Inorganic peroxides ,which are often strong oxidizing agents, are mostly active metal peroxides such as sodium peroxide (Na2O2), potassium peroxide (K2O2), etc. When encountering water or heat, they can release oxygen atom and lead to the combustion or explosion of combustible. Common inorganic peroxides include hydrogen peroxide, sodium peroxide, barium peroxide, calcium peroxide, peroxydisulfate and ammonium sulphate. Organic peroxides, such as phthalimido peroxide [(C6H5CO) 2O2] and methyl ethyl ketone peroxide [C2H5COOCH3], etc., are usually regarded as hydrogen peroxide (H-O-O-H) derivatives. And they are the initial product of the oxidation of a combustible substance and therefore extremely unstable. They are easily decomposed when encountering heat, impact, friction or shock, causing oxidation - reduction reactions and leading to self-combustion and self-explosion or the combustion and explosion of combustible materials in contact with them. In their storage and transportation, peroxides should be in intact packaging, isolated from substances with an incompatible nature, away from fire, heat, water, moisture and direct sunlight, also aloof from friction, impact and rolling. Stabilizer should be added in the storage and transportation of dibenzoyl peroxide and other organic peroxides.
The main usage of peroxide is oxidant. Hydrogen peroxide is a pollution-free oxidant, with a strong bactericidal capacity. 3% hydrogen peroxide is used as sanitizer in medicament. It’s also used for bleaching wool, silk, feathers and other fabrics in industry. In laboratory and chemical reagents production, 30% hydrogen peroxide solution is also commonly used as an oxidizing agent in the synthesis of sodium perborate and sodium carbonate for detergent. Pure hydrogen peroxide is also used as rocket fuel. Sodium peroxide is peroxide of wide usage (details at "sodium peroxide"). Peroxysulphate is used as polymerization catalyst. The complex peroxyacids of chromium, titanium and vanadium have their characteristic colors, which are commonly used in qualitative identification and quantitative determination of these metal ions. Alkaline earth metal peroxides are raw materials for the production of fireworks.
- Structure:
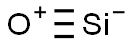
- Chemical Name:Silicon monoxide
- CAS:10097-28-6
- MF:OSi
- Structure:
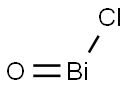
- Chemical Name:BISMUTH OXYCHLORIDE
- CAS:7787-59-9
- MF:BiClO
- Structure:

- Chemical Name:Rhodium oxide
- CAS:12036-35-0
- MF:H2ORh
- Structure:

- Chemical Name:BERYLLIUM OXIDE
- CAS:1304-56-9
- MF:BeO
- Structure:
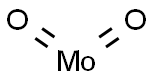
- Chemical Name:Molybdenum(IV) oxide
- CAS:18868-43-4
- MF:MoO2
- Structure:
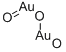
- Chemical Name:Digold trioxide
- CAS:1303-58-8
- MF:Au2O3
- Structure:

- Chemical Name:Aluminum oxide
- CAS:11092-32-3
- MF:AlO2
- Structure:
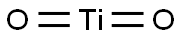
- Chemical Name:TITANIUM DIOXIDE
- CAS:1317-70-0
- MF:O2Ti
- Structure:
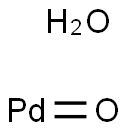
- Chemical Name:PALLADIUM(II) OXIDE
- CAS:64109-12-2
- MF:OPd
- Structure:
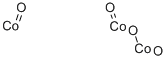
- Chemical Name:Tricobalt tetraoxide
- CAS:1308-06-1
- MF:Co3O4-2
- Structure:
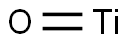
- Chemical Name:TITANIUM MONOXIDE
- CAS:12137-20-1
- MF:OTi
- Structure:
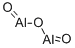
- Chemical Name:ALUMINUM OXIDE
- CAS:1302-74-5
- MF:Al2O3
- Structure:
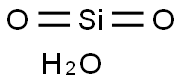
- Chemical Name:Silica glass
- CAS:10279-57-9
- MF:H2O3Si
- Structure:
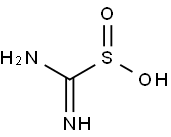
- Chemical Name:Thiourea dioxide
- CAS:1758-73-2
- MF:CH4N2O2S
- Structure:
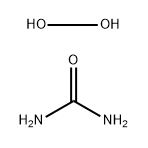
- Chemical Name:Urea hydrogen peroxide
- CAS:124-43-6
- MF:CH6N2O3
- Structure:
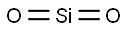
- Chemical Name:Silicon dioxide
- CAS:7631-86-9
- MF:O2Si
- Structure:
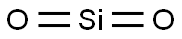
- Chemical Name:Quartz
- CAS:14808-60-7
- MF:O2Si
- Structure:
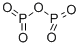
- Chemical Name:Phosphorus pentoxide
- CAS:1314-56-3
- MF:O5P2
- Chemical Name:Microsilica gel
- CAS:
- MF:
- Structure:
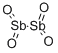
- Chemical Name:ANTIMONY (IV) OXIDE
- CAS:1332-81-6
- MF:O4Sb2
- Structure:
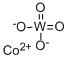
- Chemical Name:COBALT TUNGSTATE
- CAS:10101-58-3
- MF:CoO4W
- Chemical Name:High Purity Alpha Alumina(α)
- CAS:
- MF:
- Structure:
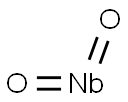
- Chemical Name:NIOBIUM (IV) OXIDE
- CAS:12034-59-2
- MF:NbO2
- Structure:
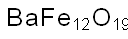
- Chemical Name:BARIUM FERRITE NANOPOWDER
- CAS:12047-11-9
- MF:BaFeH4O
- Structure:
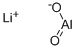
- Chemical Name:LITHIUM ALUMINATE
- CAS:12003-67-7
- MF:AlLiO2
- Structure:
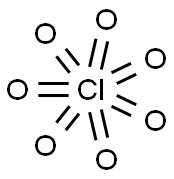
- Chemical Name:Chlorine Heptoxide
- CAS:10294-48-1
- MF:Cl2O7
- Structure:
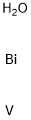
- Chemical Name:BISMUTH VANADIUM OXIDE
- CAS:14059-33-7
- MF:BiH2OV
- Chemical Name:Lanthanum cerium oxide
- CAS:
- MF:
- Structure:
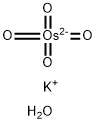
- Chemical Name:Potassium osmate(VI) dihydrate
- CAS:10022-66-9
- MF:H2KO5Os-
- Structure:
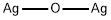
- Chemical Name:Silver oxide
- CAS:20667-12-3
- MF:Ag2O
- Structure:
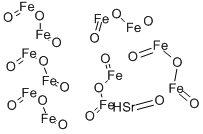
- Chemical Name:STRONTIUM FERRITE
- CAS:12023-91-5
- MF:Fe12O19Sr
- Structure:
- Chemical Name:COPPER TUNGSTATE
- CAS:13587-35-4
- MF:CuH2OW
- Structure:
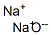
- Chemical Name:Sodium oxide
- CAS:1313-59-3
- MF:Na2O
- Structure:
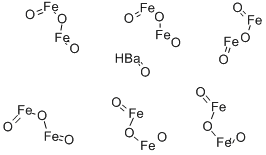
- Chemical Name:BARIUM FERRITE
- CAS:11138-11-7
- MF:BaO.6Fe2O3
- Structure:
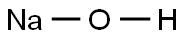
- Chemical Name:SODIUM DEUTEROXIDE
- CAS:14014-06-3
- MF:HNaO
- Structure:

- Chemical Name:Silver Hydroxide
- CAS:12673-77-7
- MF:AgHO-
- Structure:
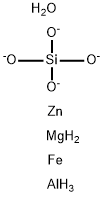
- Chemical Name:staurolite
- CAS:12182-56-8
- MF:AlFeH7MgO5SiZn-4
- Chemical Name:Iron Oxide Black
- CAS:12227-89-3
- MF:Fe3O4
- Structure:
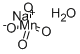
- Chemical Name:SODIUM PERMANGANATE MONOHYDRATE
- CAS:79048-36-5
- MF:H2MnNaO5
- Structure:
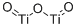
- Chemical Name:Titanium(III) oxide
- CAS:1344-54-3
- MF:O3Ti2
- Structure:
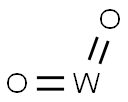
- Chemical Name:TUNGSTEN (IV) OXIDE
- CAS:12036-22-5
- MF:O2W
- Chemical Name:Cobalt green
- CAS:
- MF:
- Structure:
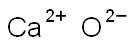
- Chemical Name:CALCIUM OXIDE
- CAS:73018-51-6
- MF:CaO
- Structure:
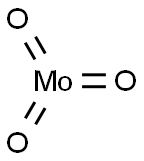
- Chemical Name:Molybdenum trioxide
- CAS:1313-27-5
- MF:MoO3
- Structure:
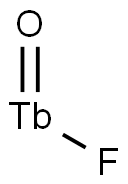
- Chemical Name:TERBIUM FLUORIDE
- CAS:21031-92-5
- MF:F3Tb
- Structure:
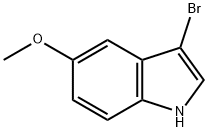
- Chemical Name:3-BROMO-5-METHOXY-1H-INDOLE
- CAS:85092-83-7
- MF:C9H8BrNO
- Structure:
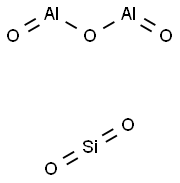
- Chemical Name:Zeolite
- CAS:1318-02-1
- MF:Al2O5Si
- Structure:
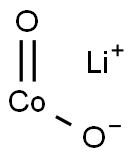
- Chemical Name:LITHIUM COBALT(III) OXIDE
- CAS:12190-79-3
- MF:CoLiO2
- Structure:
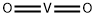
- Chemical Name:VANADIUM(IV) OXIDE
- CAS:12036-21-4
- MF:O2V
- Structure:
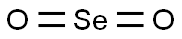
- Chemical Name:Selenium dioxide
- CAS:7446-08-4
- MF:O2Se
- Structure:
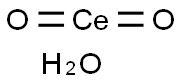
- Chemical Name:CERIUM(IV) OXIDE
- CAS:23322-64-7
- MF:CeH2O3
- Structure:
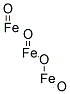
- Chemical Name:Iron oxide black
- CAS:1309-38-2
- MF:Fe3O4
- Structure:
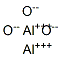
- Chemical Name:Aluminum oxide
- CAS:1344-28-1
- MF:Al2O3
- Structure:
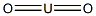
- Chemical Name:URANIUM(IV) OXIDE
- CAS:1344-57-6
- MF:O2U
- Structure:
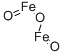
- Chemical Name:Ferric oxide
- CAS:1309-37-1
- MF:Fe2O3
- Structure:

- Chemical Name:Iron oxide
- CAS:1332-37-2
- MF:Fe2O3
- Structure:
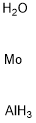
- Chemical Name:ALUMINUM MOLYBDATE
- CAS:15123-80-5
- MF:AlH5MoO
- Structure:
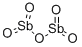
- Chemical Name:Diantimony pentoxide
- CAS:1314-60-9
- MF:O5Sb2
- Structure:
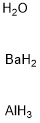
- Chemical Name:BARIUM ALUMINATE
- CAS:12004-04-5
- MF:AlBaH7O
- Structure:
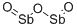
- Chemical Name:Diantimony trioxide
- CAS:1309-64-4
- MF:O3Sb2
- Structure:
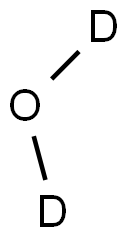
- Chemical Name:DEUTERIUM OXIDE
- CAS:7789-20-0
- MF:D2O
- Structure:
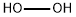
- Chemical Name:Hydrogen peroxide
- CAS:7722-84-1
- MF:H2O2
- Structure:
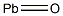
- Chemical Name:Lead monoxide
- CAS:1317-36-8
- MF:OPb
- Structure:
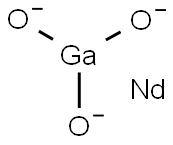
- Chemical Name:NEODYMIUM GALLIUM OXIDE
- CAS:12207-22-6
- MF:GaNdO3-3
- Structure:
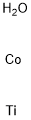
- Chemical Name:COBALT TITANATE
- CAS:12017-01-5
- MF:CoH2OTi
- Structure:
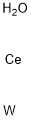
- Chemical Name:CERIUM TUNGSTATE
- CAS:13454-74-5
- MF:CeH2OW
- Structure:
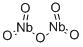
- Chemical Name:Niobium oxide
- CAS:1313-96-8
- MF:Nb2O5
- Structure:
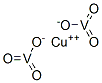
- Chemical Name:COPPER VANADATE
- CAS:14958-34-0
- MF:CuO6V2
- Structure:
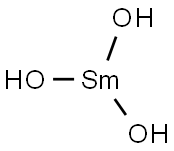
- Chemical Name:SAMARIUM(III) HYDROXIDE HYDRATE
- CAS:20403-06-9
- MF:H5O4Sm
- Structure:
- Chemical Name:BARIUM NIOBIUM OXIDE
- CAS:12009-14-2
- MF:BaH4NbO
- Structure:
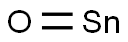
- Chemical Name:TIN(II) OXIDE
- CAS:21651-19-4
- MF:OSn
- Structure:
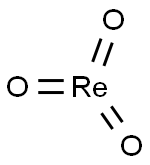
- Chemical Name:RHENIUM (VI) OXIDE
- CAS:1314-28-9
- MF:O3Re
- Structure:
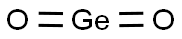
- Chemical Name:Germanium oxide
- CAS:1310-53-8
- MF:GeO2
- Structure:
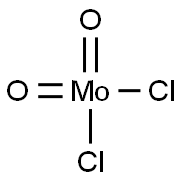
- Chemical Name:MOLYBDENUM(VI) DICHLORIDE DIOXIDE
- CAS:13637-68-8
- MF:Cl2MoO2
- Structure:
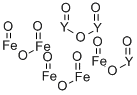
- Chemical Name:IRON YTTRIUM OXIDE
- CAS:12063-56-8
- MF:Fe5O12Y3
- Structure:
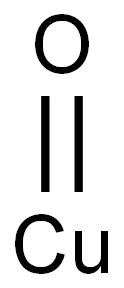
- Chemical Name:Cupric oxide
- CAS:1317-38-0
- MF:CuO
- Structure:
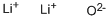
- Chemical Name:Lithium oxide
- CAS:12057-24-8
- MF:Li2O
- Structure:
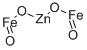
- Chemical Name:ZINC IRON OXIDE
- CAS:12063-19-3
- MF:Fe2O4Zn
- Structure:
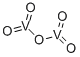
- Chemical Name:Vanadium(V) oxide
- CAS:1314-62-1
- MF:O5V2
- Structure:

- Chemical Name:Sulfuryl bromide fluoride
- CAS:13536-61-3
- MF:BrFO2S
- Structure:
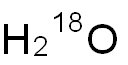
- Chemical Name:Water-18O
- CAS:14314-42-2
- MF:H2O
- Structure:

- Chemical Name:TITANIUM OXIDE
- CAS:12065-65-5
- MF:H2OTi
- Structure:
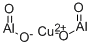
- Chemical Name:COPPER ALUMINUM OXIDE
- CAS:12042-92-1
- MF:Al2CuO4
- Structure:
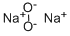
- Chemical Name:Sodium peroxide
- CAS:1313-60-6
- MF:Na2O2
- Structure:
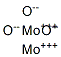
- Chemical Name:molybdenum sesquioxide
- CAS:1313-29-7
- MF:Mo2O3
- Structure:
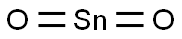
- Chemical Name:Stannic oxide
- CAS:18282-10-5
- MF:O2Sn
- Chemical Name:DIATOMACEOUS EARTH
- CAS:91053-39-3
- MF:O2Si
- Structure:
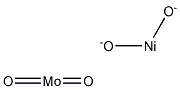
- Chemical Name:NICKEL MOLYBDATE
- CAS:14177-55-0
- MF:MoNiO4
- Structure:
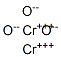
- Chemical Name:Chromium(III) oxide
- CAS:1308-38-9
- MF:Cr2O3
- Structure:
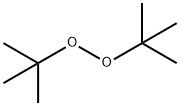
- Chemical Name:Di-tert-butyl peroxide
- CAS:110-05-4
- MF:C8H18O2
- Structure:
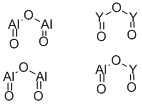
- Chemical Name:YTTRIUM ALUMINUM OXIDE
- CAS:12005-21-9
- MF:Al5O12Y3
- Structure:
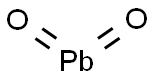
- Chemical Name:Lead dioxide
- CAS:1309-60-0
- MF:O2Pb
- Structure:
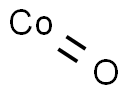
- Chemical Name:Cobalt oxide
- CAS:1307-96-6
- MF:CoO
- Structure:
- Chemical Name:CERIUM ZIRCONIUM OXIDE
- CAS:53169-24-7
- MF:CeH2OZr
- Structure:
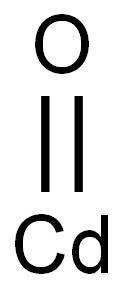
- Chemical Name:Cadmium oxide
- CAS:1306-19-0
- MF:CdO
- Structure:

- Chemical Name:AMMONIUM TUNGSTATE
- CAS:11140-77-5
- MF:H6NOW+
- Chemical Name:EMERY
- CAS:12415-34-8
- MF:
- Structure:
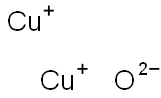
- Chemical Name:Cuprous oxide
- CAS:1317-39-1
- MF:Cu2O
- Structure:
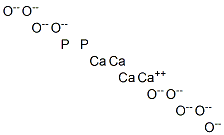
- Chemical Name:tetracalcium diphosphorus nonaoxide
- CAS:1306-01-0
- MF:Ca4H6O9P2-16