Metal peroxide
Metal peroxide can be used as oxygen regeneration agent in spacecraft or submarine, such as sodium peroxide (Na2O2)react with CO2 exhaled by human to generate oxygen at room temperature, the reaction is: 2Na2O2 +2 CO2 = 2Na2CO3 + O2.
Metal peroxides can be regarded as the two hydrogen atoms in hydrogen peroxide were replaced by metal atoms, alkali metal, alkaline earth metal form into ions peroxides; the peroxides of zinc, cadmium, mercury peroxides are covalent; and the peroxide of magnesium is between the two.
Click on the specific product, view the latest prices of the products, information, serving information
- Structure:
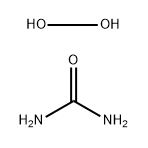
- Chemical Name:Urea hydrogen peroxide
- CAS:124-43-6
- MF:CH6N2O3
- Structure:
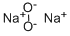
- Chemical Name:Sodium peroxide
- CAS:1313-60-6
- MF:Na2O2
- Structure:
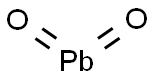
- Chemical Name:Lead dioxide
- CAS:1309-60-0
- MF:O2Pb
- Structure:
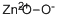
- Chemical Name:Zinc peroxide
- CAS:1314-22-3
- MF:O2Zn
- Structure:
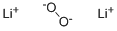
- Chemical Name:Lithium peroxide
- CAS:12031-80-0
- MF:Li2O2
- Structure:
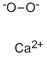
- Chemical Name:Calcium peroxide
- CAS:1305-79-9
- MF:CaO2
- Structure:
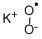
- Chemical Name:Potassium superoxide
- CAS:12030-88-5
- MF:KO2*
- Structure:

- Chemical Name:Nickel peroxide
- CAS:12035-36-8
- MF:NiO2
- Structure:
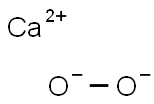
- Chemical Name:CALCIUM PEROXIDE, 75%, REM CALCIUM HYDRO X-IDE & CALCIUM OXIDE, POWDER, -200 MESH
- CAS:78403-22-2
- MF:CaO2
- Structure:

- Chemical Name:Magnesium peroxide
- CAS:1335-26-8
- MF:MgO2
- Structure:

- Chemical Name:SODIUM PEROXIDE, EXTRA PURE
- CAS:
- MF:Na2O2