Oxides and peroxides
Oxide is an inorganic compound consisting of two elements, one of which is oxygen. Except helium, neon, krypton and other rare elements, all of other elements can form an oxide with oxygen. According to their acidity, oxides are generally divided into alkaline oxides, acid oxides and amphoteric oxides. Basic oxide is capable of forming a salt with an acid, acidic oxide is capable of reacting with the alkali solution to produce a salt, and the amphoteric oxide is capable of both above. Some oxides such as carbon monoxide (CO), nitric oxide (NO), etc., are known as neutral oxides for they react neither with an acid nor with a base. Oxides are widely distributed in nature, such carbon dioxide in the air and silicon dioxide (SiO2), the main component of quartz sand. And the principal component of hematite, which is a raw material for ion, is ferric oxide.
The main applications of oxides: ① catalyst, ② inorganic pigments, ③ magnetic material, ④ optical material, ⑤ superconducting material, ⑥ chemical separation.
Peroxide, also known as superoxide, is a class of compounds consisting of peroxide groups (-O-O-). Peroxide can be inorganic or organic . Inorganic peroxides ,which are often strong oxidizing agents, are mostly active metal peroxides such as sodium peroxide (Na2O2), potassium peroxide (K2O2), etc. When encountering water or heat, they can release oxygen atom and lead to the combustion or explosion of combustible. Common inorganic peroxides include hydrogen peroxide, sodium peroxide, barium peroxide, calcium peroxide, peroxydisulfate and ammonium sulphate. Organic peroxides, such as phthalimido peroxide [(C6H5CO) 2O2] and methyl ethyl ketone peroxide [C2H5COOCH3], etc., are usually regarded as hydrogen peroxide (H-O-O-H) derivatives. And they are the initial product of the oxidation of a combustible substance and therefore extremely unstable. They are easily decomposed when encountering heat, impact, friction or shock, causing oxidation - reduction reactions and leading to self-combustion and self-explosion or the combustion and explosion of combustible materials in contact with them. In their storage and transportation, peroxides should be in intact packaging, isolated from substances with an incompatible nature, away from fire, heat, water, moisture and direct sunlight, also aloof from friction, impact and rolling. Stabilizer should be added in the storage and transportation of dibenzoyl peroxide and other organic peroxides.
The main usage of peroxide is oxidant. Hydrogen peroxide is a pollution-free oxidant, with a strong bactericidal capacity. 3% hydrogen peroxide is used as sanitizer in medicament. It’s also used for bleaching wool, silk, feathers and other fabrics in industry. In laboratory and chemical reagents production, 30% hydrogen peroxide solution is also commonly used as an oxidizing agent in the synthesis of sodium perborate and sodium carbonate for detergent. Pure hydrogen peroxide is also used as rocket fuel. Sodium peroxide is peroxide of wide usage (details at "sodium peroxide"). Peroxysulphate is used as polymerization catalyst. The complex peroxyacids of chromium, titanium and vanadium have their characteristic colors, which are commonly used in qualitative identification and quantitative determination of these metal ions. Alkaline earth metal peroxides are raw materials for the production of fireworks.
- Structure:
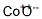
- Chemical Name:Cobalt oxide
- CAS:11104-61-3
- MF:CoO
- Structure:
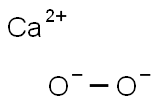
- Chemical Name:CALCIUM PEROXIDE, 75%, REM CALCIUM HYDRO X-IDE & CALCIUM OXIDE, POWDER, -200 MESH
- CAS:78403-22-2
- MF:CaO2
- Structure:

- Chemical Name:BARIUM COPPER OXIDE (1-1), 99.9% (METALS BASIS)
- CAS:
- MF:BaCuO2
- Structure:
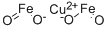
- Chemical Name:COPPER IRON OXIDE NANOPOWDER 98.5%
- CAS:12018-79-0
- MF:Cu.2FeO2
- Chemical Name:Cupric oxide asbestos
- CAS:
- MF:
- Structure:

- Chemical Name:Lanthanum strontium cobalt oxide
- CAS:108916-09-2
- MF:CoH4LaOSr
- Chemical Name:aluminium oxide sol
- CAS:
- MF:[Al2(OH)nX6-n]m
- Chemical Name:Ferric oxide,nanometre
- CAS:
- MF:Fe2O3
- Chemical Name:Sweetening agent for ironicoxide
- CAS:
- MF:
- Chemical Name:Silicon dioxide Nanopowder
- CAS:
- MF:
- Chemical Name:Magnesium hydroxide,activated
- CAS:
- MF:Mg(OH)2
- Chemical Name:iron oxide blue
- CAS:
- MF:
- Chemical Name:Hafnium(Ⅳ) oxide
- CAS:
- MF:HfO2
- Structure:
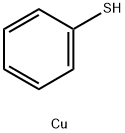
- Chemical Name:Copper(I) thiophenoxide, 95%
- CAS:34012-88-9
- MF:C6H6CuS
- Structure:

- Chemical Name:HAFNIUM DINITRATE OXIDE
- CAS:
- MF:H4HfN2O9
- Chemical Name:ZIRCONIUM (IV) OXIDE CEMENT
- CAS:
- MF:
- Chemical Name:Cerium Rich Hydrate
- CAS:
- MF:
- Structure:
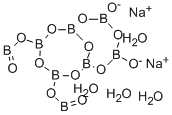
- Chemical Name:DISODIUM OCTABORATE TETRAHYDRATE
- CAS:12008-41-2
- MF:B8H8Na2O17
- Structure:

- Chemical Name:SODIUM PEROXIDE, EXTRA PURE
- CAS:
- MF:Na2O2
- Chemical Name:γ-Aluminum oxide
- CAS:68389-42-4
- MF:Al2O3
- Structure:

- Chemical Name:BARIUM CALCIUM COPPER OXIDE (2-1-2), 99.9% (METALS BASIS)
- CAS:
- MF:BaCaCuO3
- Chemical Name:Zinc oxide of direct method
- CAS:
- MF:
- Structure:
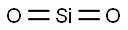
- Chemical Name:SILICA
- CAS:14464-46-1
- MF:O2Si
- Structure:
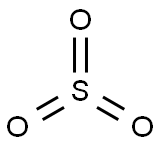
- Chemical Name:Sulfur trioxide
- CAS:7446-11-9
- MF:O3S
- Structure:

- Chemical Name:Yttrium chlorid hexahydrate
- CAS:
- MF:Cl3H12O6Y
- Chemical Name:Erbium(Ⅲ)oxide
- CAS:
- MF:Er2O3
- Chemical Name:Thulium(Ⅲ)chloride heptahydrate
- CAS:
- MF:
- Chemical Name:Hydrous zirconium oxide
- CAS:
- MF:ZrO2·nH2O
- Chemical Name:Micaceous Iron Oxide
- CAS:
- MF:α-Fe2O3
- Chemical Name:activated manganese dioxide
- CAS:
- MF:
- Chemical Name:OSMIUM (VIII) OXIDE, MICROENCAPSULATED IN A STYRENE POLYMER (~10%OSO4)
- CAS:
- MF:OsO4
- Chemical Name:ZNO 99.2900
- CAS:
- MF:C2O4Zn.2H2O
- Structure:
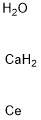
- Chemical Name:CALCIUM CERIUM OXIDE
- CAS:170087-78-2
- MF:CaCeH4O
- Chemical Name:Activated calcium oxide
- CAS:
- MF:CaO
- Structure:

- Chemical Name:Magnesium peroxide
- CAS:1335-26-8
- MF:MgO2
- Chemical Name:PLATINUM(Ⅳ) OXIDE
- CAS:
- MF:PtO2·xH2O
- Structure:

- Chemical Name:MAGNESIUM OXIDE SUBSTRATE, 10X10X0.5MM, POLISHED ONE SIDE, 111 ORIENTATION
- CAS:
- MF:MgO
- Chemical Name:Zinc oxide of indirect method
- CAS:
- MF:
- Structure:
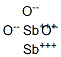
- Chemical Name:Antimony(III) Oxide
- CAS:1327-33-9
- MF:O3Sb2
- Structure:
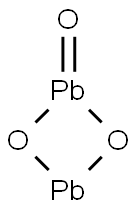
- Chemical Name:LEAD SESQUIOXIDE
- CAS:1314-27-8
- MF:O3Pb2
- Structure:

- Chemical Name:NICKEL ZINC IRON OXIDE NANOPOWDER 99+%
- CAS:12645-50-0
- MF:FeH2NiOZn
- Structure:
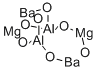
- Chemical Name:Aluminum barium magnesium oxide
- CAS:63774-55-0
- MF:Al2Ba2Mg2O7
- Structure:
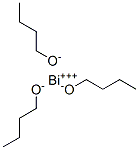
- Chemical Name:bismuth III n-buthoxide
- CAS:90520-71-1
- MF:C12H27BiO3
- Structure:
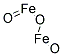
- Chemical Name:Pigment Yellow 42
- CAS:51274-00-1
- MF:Fe2O3
- Structure:
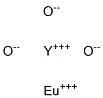
- Chemical Name:Yttrium europium oxide
- CAS:
- MF:EuO3Y
- Chemical Name:ZNO 99.7100
- CAS:
- MF:
- Structure:
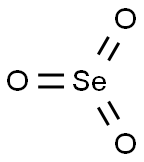
- Chemical Name:Selenium trioxide
- CAS:13768-86-0
- MF:O3Se