Organic reagents
From early AD to mid-19 century, people mainly use the natural organic substance (such as animal and plant extracts) for qualitative analysis or quantitative analysis. From the second half of the 19th century to the 1920s, it had begun to appear of artificially synthetic organic reagent such as using potassium acetate xanthan for test of nickel, copper, and molybdenum; using morin for test of aluminum; using diazo coupling reaction for the detection of Nitrite; using α-β-nitroso naphthol for detection of cobalt; using dimethyglyoxime for nickel test.
After the proposal of the special-effects group in the 1930s and the proposal of theoretical analysis of functional groups theory in 1950s, people had carried out large-scale screen of organic reagents in search of special-effects analysis groups for different ions and had successfully synthesized a lot of agents of practical value (such as copper reagents, new copper agent, cadmium reagents, beryllium reagent, thorium reagents, etc.). Before the 1950s, the complex compound, in analytic chemistry, is mainly used in the aspects of the precipitation reaction of a binary chelate for the qualitative detection, precipitate isolation and gravimetric separation and other aspects. In the early 1950s and 1960, it is mainly in the form of complexometric titration. From the beginning of the late 1960s, the main focus has been moved to the photometric analysis. Meanwhile, it has been also developed of chelate organic solvent extraction.
8-hydroxy quinoline, hydrazones, oximes, hydroxamic acids, polyphenols, 1, 10-phenanthroline, phosphorus-oxygen extractant, β- diketones, triphenylmethane acid dye, xanthene dyes and azo dyes and other agents has all been widely studied and applied. On the other hand, the structural theoretical research (such as electronic effects, spatial effects and substituent effects) of the organic reagent can further facilitate the development of new-type organic reagents such as heterocyclic azo reagent. During this period, it has been also developed of monoazo chromotropic acid-type and disazo chromotropic acid-type reagent. During the mid-1970s, the chelating resin and adsorbents have achieved rapid development. Since the 1980s, Chinese researchers of analytical chemistry have made progress in the field of the synthesis and application of asymmetric disazo chromotropic acid.
Organic chemical reagents have wide application in analytic chemistry and are mainly used in solvents, precipitation agents, complexing agents, indicator, coloring reagent and surface active agent, etc. In order to adapt to a variety of analytical needs, sometimes it is required to purify certain reagents. For example, liquid organic reagent is often purified by distillation while solid substance is often purified through crystallization and sublimation. Substance having a high vapor pressure, namely having low purification boiling point and undergoing no decomposition under boiling temperature commonly applied atmospheric distillation; for less volatile and water-insoluble substance, namely having high purification point or undergoing decomposition under boiling temperature, we can apply vacuum distillation or steam distillation for purification of it.
Distillation is a commonly used method for laboratory purification of organic reagent. Conventional distillation apparatus generally comprises distiller, condenser and receiver, three parts with auxiliary section also containing heater, a thermometer, a wetted tube and cork.
- Structure:
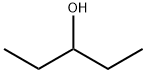
- Chemical Name:3-Pentanol
- CAS:584-02-1
- MF:C5H12O
- Structure:
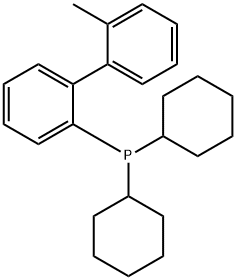
- Chemical Name:2-(Dicyclohexylphosphino)-2'-methylbiphenyl
- CAS:251320-86-2
- MF:C25H33P
- Structure:
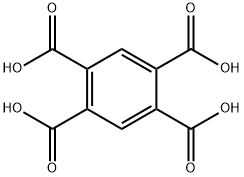
- Chemical Name:Pyromellitic acid
- CAS:89-05-4
- MF:C10H6O8
- Structure:
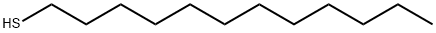
- Chemical Name:1-Dodecanethiol
- CAS:112-55-0
- MF:C12H26S
- Structure:
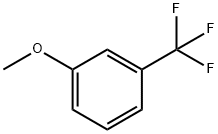
- Chemical Name:3-(Trifluoromethyl)anisole
- CAS:454-90-0
- MF:C8H7F3O
- Structure:
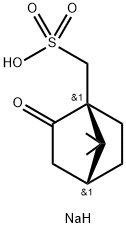
- Chemical Name:Sodium (+)-10-camphorsulfonate
- CAS:21791-94-6
- MF:C10H17NaO4S
- Structure:
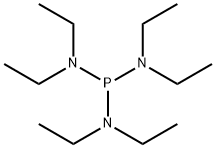
- Chemical Name:TRIS(DIETHYLAMINO)PHOSPHINE
- CAS:2283-11-6
- MF:C12H30N3P
- Structure:
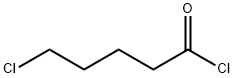
- Chemical Name:5-Chlorovaleryl chloride
- CAS:1575-61-7
- MF:C5H8Cl2O
- Structure:
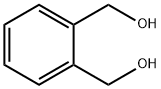
- Chemical Name:1,2-Benzenedimethanol
- CAS:612-14-6
- MF:C8H10O2
- Structure:
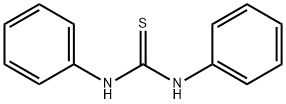
- Chemical Name:1,3-Diphenyl-2-thiourea
- CAS:102-08-9
- MF:C13H12N2S
- Structure:
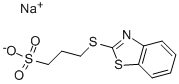
- Chemical Name:Sodium 3-(benzothiazol-2-ylthio)-1-propanesulfonate
- CAS:49625-94-7
- MF:C10H10NNaO3S3
- Structure:
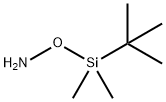
- Chemical Name:O-(TERT-BUTYLDIMETHYLSILYL)HYDROXYLAMINE
- CAS:41879-39-4
- MF:C6H17NOSi
- Structure:
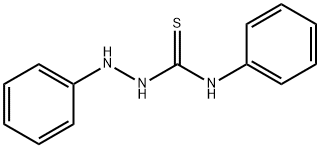
- Chemical Name:1,4-DIPHENYL-3-THIOSEMICARBAZIDE
- CAS:1768-59-8
- MF:C13H13N3S
- Structure:
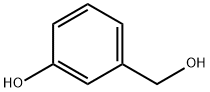
- Chemical Name:3-Hydroxybenzyl alcohol
- CAS:620-24-6
- MF:C7H8O2
- Structure:
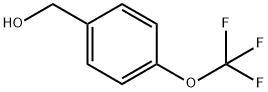
- Chemical Name:4-(Trifluoromethoxy)benzyl alcohol
- CAS:1736-74-9
- MF:C8H7F3O2
- Structure:
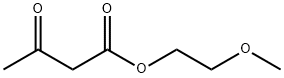
- Chemical Name:2-Methoxyethyl acetoacetate
- CAS:22502-03-0
- MF:C7H12O4
- Structure:
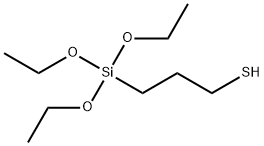
- Chemical Name:3-Mercaptopropyltriethoxysilane
- CAS:14814-09-6
- MF:C9H22O3SSi
- Structure:
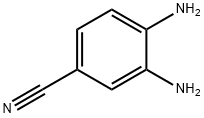
- Chemical Name:3,4-Diaminobenzonitrile
- CAS:17626-40-3
- MF:C7H7N3
- Structure:
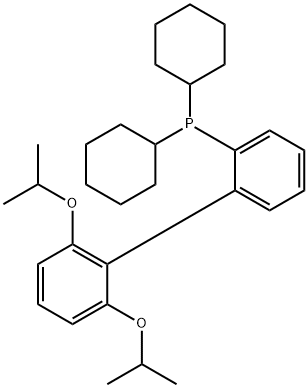
- Chemical Name:2-DICYCLOHEXYLPHOSPHINO-2',6'-DIISOPROPOXYBIPHENYL
- CAS:787618-22-8
- MF:C30H43O2P
- Structure:
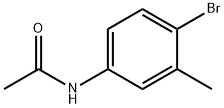
- Chemical Name:4'-BROMO-3'-METHYLACETANILIDE
- CAS:90914-81-1
- MF:C9H10BrNO
- Structure:
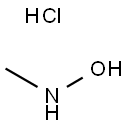
- Chemical Name:N-Methylhydroxylamine hydrochloride
- CAS:4229-44-1
- MF:CH6ClNO
- Structure:
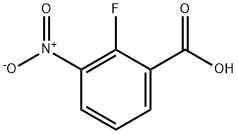
- Chemical Name:2-Fluoro-3-nitrobenzoic acid
- CAS:317-46-4
- MF:C7H4FNO4
- Structure:
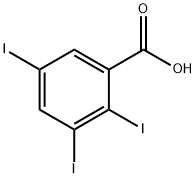
- Chemical Name:2,3,5-Triiodobenzoic acid
- CAS:88-82-4
- MF:C7H3I3O2
- Structure:
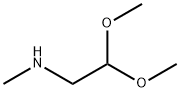
- Chemical Name:Methylaminoacetaldehyde dimethyl acetal
- CAS:122-07-6
- MF:C5H13NO2
- Structure:
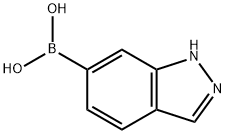
- Chemical Name:6-INDAZOLYBORONIC ACID
- CAS:885068-10-0
- MF:C7H7BN2O2
- Structure:
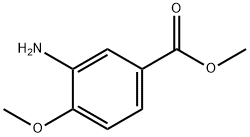
- Chemical Name:Methyl 3-amino-4-methoxybenzoate
- CAS:24812-90-6
- MF:C9H11NO3
- Structure:
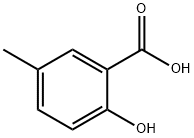
- Chemical Name:5-Methylsalicylic acid
- CAS:89-56-5
- MF:C8H8O3
- Structure:
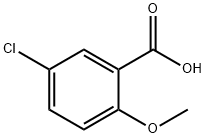
- Chemical Name:5-Chloro-2-methoxybenzoic acid
- CAS:3438-16-2
- MF:C8H7ClO3
- Structure:
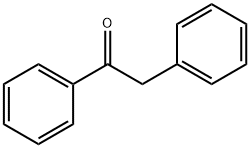
- Chemical Name:2-Phenylacetophenone
- CAS:451-40-1
- MF:C14H12O
- Structure:
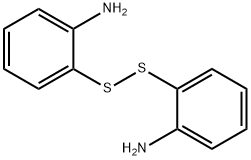
- Chemical Name:2,2'-Diaminodiphenyl disulphide
- CAS:1141-88-4
- MF:C12H12N2S2
- Structure:
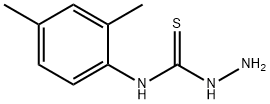
- Chemical Name:4-(2,4-DIMETHYLPHENYL)-3-THIOSEMICARBAZIDE
- CAS:66298-09-7
- MF:C9H13N3S
- Structure:
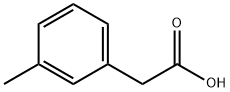
- Chemical Name:3-Methylphenylacetic acid
- CAS:621-36-3
- MF:C9H10O2
- Structure:
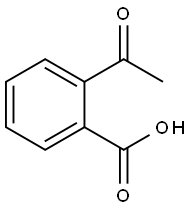
- Chemical Name:2-Acetylbenzoic acid
- CAS:577-56-0
- MF:C9H8O3
- Structure:

- Chemical Name:(TRIMETHYLSILYL)DIAZOMETHANE
- CAS:18107-18-1
- MF:C4H10N2Si
- Structure:
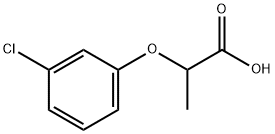
- Chemical Name:2-(3-Chlorophenoxy)-propionic acid
- CAS:101-10-0
- MF:C9H9ClO3
- Structure:
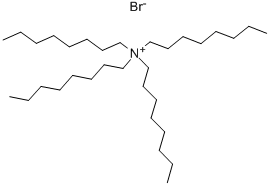
- Chemical Name:Tetraoctylammonium bromide
- CAS:14866-33-2
- MF:C32H68BrN
- Structure:
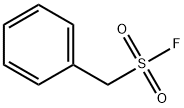
- Chemical Name:Phenylmethylsulfonyl fluoride
- CAS:329-98-6
- MF:C7H7FO2S
- Structure:
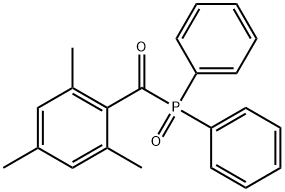
- Chemical Name:Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide
- CAS:75980-60-8
- MF:C22H21O2P
- Structure:
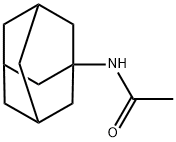
- Chemical Name:N-(1-Adamantyl)acetamide
- CAS:880-52-4
- MF:C12H19NO
- Structure:
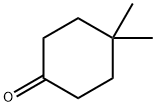
- Chemical Name:4 4-DIMETHYLCYCLOHEXANONE 97
- CAS:4255-62-3
- MF:C8H14O
- Structure:
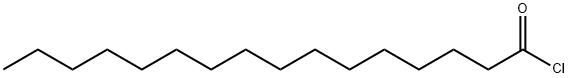
- Chemical Name:Palmitoyl chloride
- CAS:112-67-4
- MF:C16H31ClO
- Structure:

- Chemical Name:TERT-NONYL MERCAPTAN
- CAS:25360-10-5
- MF:C9H20S
- Structure:
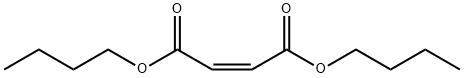
- Chemical Name:n-Butyl fumarate
- CAS:105-76-0
- MF:C12H20O4
- Structure:
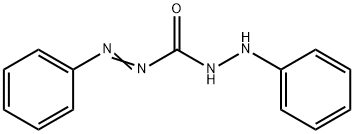
- Chemical Name:DIPHENYLCARBAZONE
- CAS:538-62-5
- MF:C13H12N4O
- Structure:
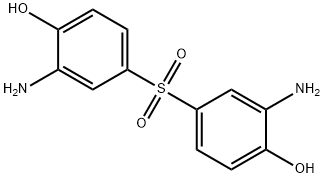
- Chemical Name:3,3'-Diamino-4,4'-dihydroxydiphenyl sulfone
- CAS:7545-50-8
- MF:C12H12N2O4S
- Structure:
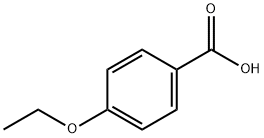
- Chemical Name:4-Ethoxybenzoic acid
- CAS:619-86-3
- MF:C9H10O3
- Structure:
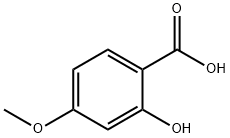
- Chemical Name:4-Methoxysalicylic acid
- CAS:2237-36-7
- MF:C8H8O4
- Structure:
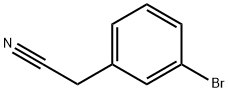
- Chemical Name:3-Bromophenylacetonitrile
- CAS:31938-07-5
- MF:C8H6BrN
- Structure:
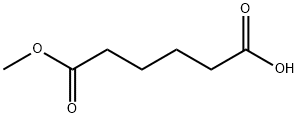
- Chemical Name:Monomethyl adipate
- CAS:627-91-8
- MF:C7H12O4
- Structure:
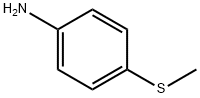
- Chemical Name:4-(Methylmercapto)aniline
- CAS:104-96-1
- MF:C7H9NS
- Structure:
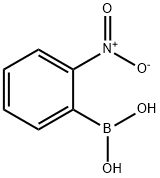
- Chemical Name:2-Nitrophenylboronic acid
- CAS:5570-19-4
- MF:C6H6BNO4
- Structure:
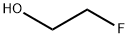
- Chemical Name:2-Fluoroethanol
- CAS:371-62-0
- MF:C2H5FO
- Structure:
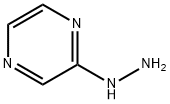
- Chemical Name:2-Hydrazinopyrazine
- CAS:54608-52-5
- MF:C4H6N4
- Structure:
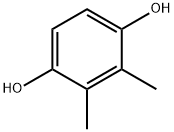
- Chemical Name:2,3-Dimethylhydroquinone
- CAS:608-43-5
- MF:C8H10O2
- Structure:
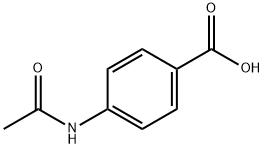
- Chemical Name:4-acetamidobenzoic acid
- CAS:556-08-1
- MF:C9H9NO3
- Structure:
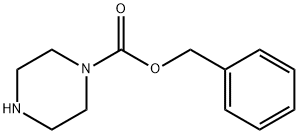
- Chemical Name:BENZYL 1-PIPERAZINECARBOXYLATE
- CAS:31166-44-6
- MF:C12H16N2O2
- Structure:
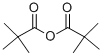
- Chemical Name:Trimethylacetic anhydride
- CAS:1538-75-6
- MF:C10H18O3
- Structure:
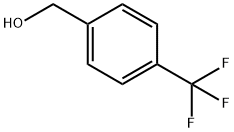
- Chemical Name:4-(Trifluoromethyl)benzyl alcohol
- CAS:349-95-1
- MF:C8H7F3O
- Structure:
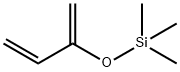
- Chemical Name:2-(Trimethylsiloxy)-1,3-butadiene
- CAS:38053-91-7
- MF:C7H14OSi
- Structure:
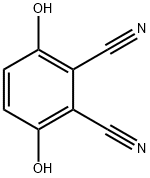
- Chemical Name:3,6-Dihydroxyphthalonitrile
- CAS:4733-50-0
- MF:C8H4N2O2
- Structure:
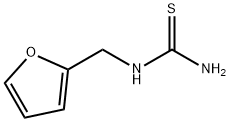
- Chemical Name:1-(2-FURFURYL)-2-THIOUREA
- CAS:56541-07-2
- MF:C6H8N2OS
- Structure:
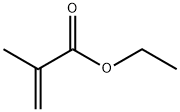
- Chemical Name:Ethyl methacrylate
- CAS:97-63-2
- MF:C6H10O2
- Structure:
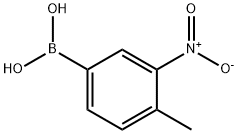
- Chemical Name:4-Methyl-3-nitrophenylboronic acid
- CAS:80500-27-2
- MF:C7H8BNO4
- Structure:
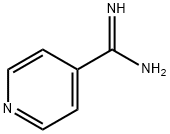
- Chemical Name:4-PYRIDINECARBOXAMIDINE
- CAS:33278-46-5
- MF:C6H7N3
- Structure:
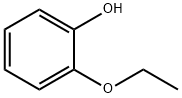
- Chemical Name:2-Ethoxyphenol
- CAS:94-71-3
- MF:C8H10O2
- Structure:
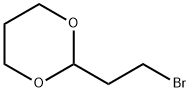
- Chemical Name:2-(2-Bromoethyl)-1,3-dioxane
- CAS:33884-43-4
- MF:C6H11BrO2
- Structure:
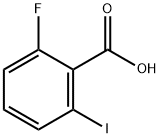
- Chemical Name:2-FLUORO-6-IODOBENZOIC ACID
- CAS:111771-08-5
- MF:C7H4FIO2
- Structure:
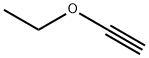
- Chemical Name:Ethoxyethyne
- CAS:927-80-0
- MF:C4H6O
- Structure:
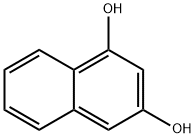
- Chemical Name:1,3-DIHYDROXYNAPHTHALENE
- CAS:132-86-5
- MF:C10H8O2
- Structure:
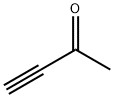
- Chemical Name:3-Butyn-2-one
- CAS:1423-60-5
- MF:C4H4O
- Structure:
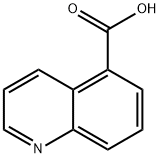
- Chemical Name:Quinoline-5-carboxylic acid
- CAS:7250-53-5
- MF:C10H7NO2
- Structure:
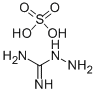
- Chemical Name:Aminoguanidinium sulphate
- CAS:1068-42-4
- MF:CH6N4.H2O4S
- Structure:
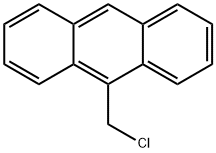
- Chemical Name:9-(Chloromethyl)anthracene
- CAS:24463-19-2
- MF:C15H11Cl
- Structure:
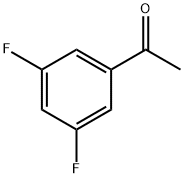
- Chemical Name:3',5'-Difluoroacetophenone
- CAS:123577-99-1
- MF:C8H6F2O
- Structure:
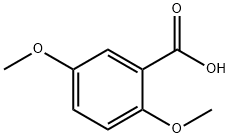
- Chemical Name:2,5-Dimethoxybenzoic acid
- CAS:2785-98-0
- MF:C9H10O4
- Structure:
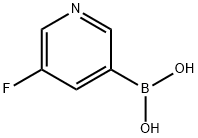
- Chemical Name:5-Fluoropyridin-3-ylboronic acid
- CAS:872041-86-6
- MF:C5H5BFNO2
- Structure:
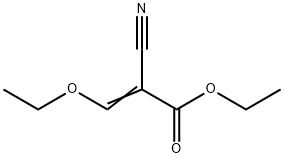
- Chemical Name:Ethyl (ethoxymethylene)cyanoacetate
- CAS:94-05-3
- MF:C8H11NO3
- Structure:
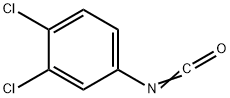
- Chemical Name:Isocyanic acid 3,4-dichlorophenyl ester
- CAS:102-36-3
- MF:C7H3Cl2NO
- Structure:
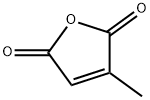
- Chemical Name:Citraconic anhydride
- CAS:616-02-4
- MF:C5H4O3
- Structure:
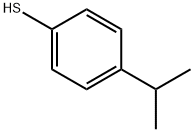
- Chemical Name:(4-Isopropyl)thiophenol
- CAS:4946-14-9
- MF:C9H12S
- Structure:
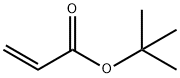
- Chemical Name:tert-Butyl acrylate
- CAS:1663-39-4
- MF:C7H12O2
- Structure:

- Chemical Name:Carbon
- CAS:7440-44-0
- MF:C
- Structure:
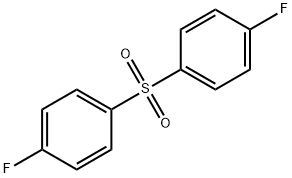
- Chemical Name:4-Fluorophenyl sulfone
- CAS:383-29-9
- MF:C12H8F2O2S
- Structure:
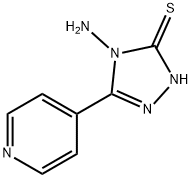
- Chemical Name:4-AMINO-5-(4-PYRIDYL)-4 H-1,2,4-TRIAZOLE-3-THIOL
- CAS:36209-51-5
- MF:C7H7N5S
- Structure:
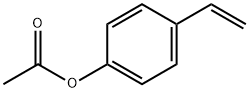
- Chemical Name:4-Acetoxystyrene
- CAS:2628-16-2
- MF:C10H10O2
- Structure:
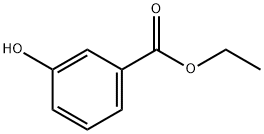
- Chemical Name:Ethyl 3-hydroxybenzoate
- CAS:7781-98-8
- MF:C9H10O3
- Structure:
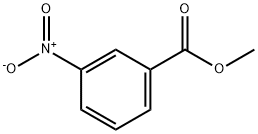
- Chemical Name:Methyl 3-nitrobenzoate
- CAS:618-95-1
- MF:C8H7NO4
- Structure:
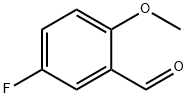
- Chemical Name:5-FLUORO-2-METHOXYBENZALDEHYDE
- CAS:19415-51-1
- MF:C8H7FO2
- Structure:
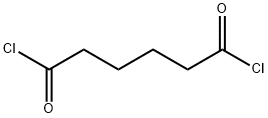
- Chemical Name:Adipoyl chloride
- CAS:111-50-2
- MF:C6H8Cl2O2
- Structure:
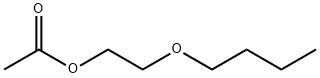
- Chemical Name:2-Butoxyethyl acetate
- CAS:112-07-2
- MF:C8H16O3
- Structure:
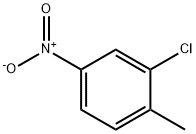
- Chemical Name:2-Chloro-4-nitrotoluene
- CAS:121-86-8
- MF:C7H6ClNO2
- Structure:
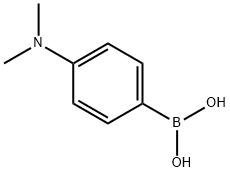
- Chemical Name:4-(Dimethylamino)phenylboronic acid
- CAS:28611-39-4
- MF:C8H12BNO2
- Structure:
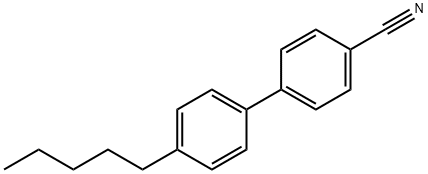
- Chemical Name:4-Cyano-4'-pentylbiphenyl
- CAS:40817-08-1
- MF:C18H19N
- Structure:
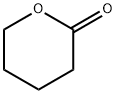
- Chemical Name:delta-Valerolactone
- CAS:542-28-9
- MF:C5H8O2
- Structure:
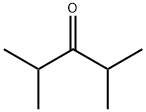
- Chemical Name:2,4-Dimethyl-3-pentanone
- CAS:565-80-0
- MF:C7H14O
- Structure:
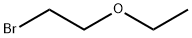
- Chemical Name:2-Bromoethyl ethyl ether
- CAS:592-55-2
- MF:C4H9BrO
- Structure:
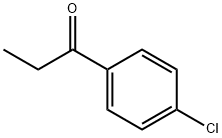
- Chemical Name:4'-Chloropropiophenone
- CAS:6285-05-8
- MF:C9H9ClO
- Structure:
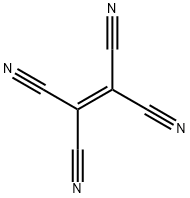
- Chemical Name:Tetracyanoethylene
- CAS:670-54-2
- MF:C6N4
- Structure:
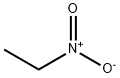
- Chemical Name:Nitroethane
- CAS:79-24-3
- MF:C2H5NO2
- Structure:
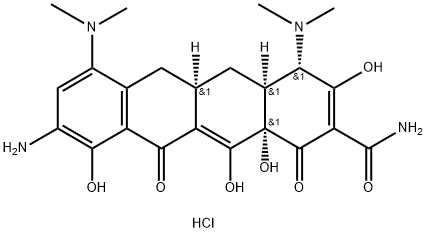
- Chemical Name:9-Amino minocycline hydrochloride
- CAS:149934-21-4
- MF:C23H29ClN4O7