Sulfone, sulfoxide compound
Sulfone is the general name for compounds formed by the combination of sulfonyl and hydrocarbyl, of which the general formula is R-SO2-R. The hydrocarbyl groups (R) can be identical or different, such as dimethyl sulfone (CH3 • SO2 • CH3), ethyl phenyl sulphone (C6H5 • SO2 • C2H5), diethyl sulfone, diphenyl sulfone, sulfolane, bisphenol S, etc. The sulfur in sulfones is of high valence; and sulfone is a stable crystalline organic compound which can’t be reduced into R2S. Sulfolane, dimethyl sulfone and bisphenol S are the most important sulfones in the industry. Sulfone is generally colorless and odorless stable solid. Lower carbon sulfone is soluble in water and most organic solvents. Sulfone can be prepared generally by the oxidation of thioether, the alkylation of sulfonate, the Diels-Alder reaction of sulfur dioxide or the reaction of aromatic hydrocarbons with sulfoxide chloride, etc.
Sulfone is widely used in pharmaceutical, plastics and basic organic synthesis industry. For example, solasulfone and dapsone are drugs for leprosy; bisphenol A polysulfone is a kind of plastic with excellent properties and sulfolane is an excellent solvent which can be used to clean industrial gases such as the removal of carbon dioxide and hydrogen sulfide; it’s also used as a solvent in the polymerization of acrylonitrile as well as in the extraction of aromatics from petroleum. Some sulfones have sedative and hypnotic effects, but with side effects. In addition to a heat resistance, oxidation resistance and light stability of bisphenol S, the hydroxyl in it has strong acidity because of the strong electron-withdrawing of the sulfonyl. Bisphenol S is mainly used as a fixative as well as the material of leather tanning agents, dyes, heat-resistant engineering plastics and crosslinking agents. It also can be used as a substitute for bisphenol A as the raw material of polycarbonate, epoxy resin, polyester resin and phenol resin.
Sulfoxide is the general name for compounds formed by the combination of sulfinyl and hydrocarbyl (R), such as dimethyl sulfoxide, diethyl sulfoxide, benzyl phenyl sulfoxide. The oxygen atom in sulfoxide is in an anion state with a strong polarity and a strong oxidizability. Sulfoxides may be optically active. They are solid at low temperatures and generally soluble in water, alcohol and ether. Sulfoxide can be reduced into sulfide, oxidized into the sulfone and salified with nitrate. It can be prepared generally by the oxidation of sulfide or the Friedel-Crafts reaction of an aromatic hydrocarbon with thionyl chloride.
Sulfoxide can take part in many important reactions.
1, The α-carbanion in sulfinyl can easily react with haloalkane, carbonyl compounds and olefins due to its high reactivity;
2, Sulfoxide along with acid anhydride can induce the Pummerer rearrangement;
3, Olefins and sulfinic acid can be generated after the thermal decomposition of sulfoxide with a β site containing hydrogen.
- Structure:
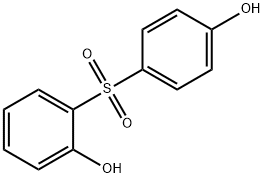
- Chemical Name:2,4'-DIHYDROXYDIPHENYL SULFONE
- CAS:5397-34-2
- MF:C12H10O4S
- Structure:
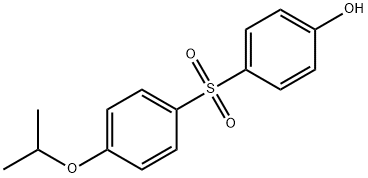
- Chemical Name:4-Hydroxy-4'-isopropoxydiphenylsulfone
- CAS:95235-30-6
- MF:C15H16O4S
- Structure:
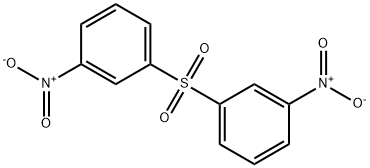
- Chemical Name:3-Nitrophenyl sulphone
- CAS:1228-53-1
- MF:C12H8N2O6S
- Structure:
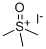
- Chemical Name:Trimethylsulfoxonium iodide
- CAS:1774-47-6
- MF:C3H9IOS
- Structure:
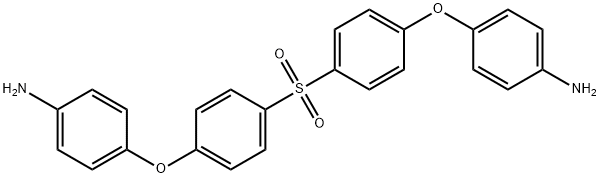
- Chemical Name:Bis[4-(4-aminophenoxy)phenyl]sulfone
- CAS:13080-89-2
- MF:C24H20N2O4S
- Structure:
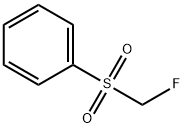
- Chemical Name:Fluoromethyl phenyl sulfone
- CAS:20808-12-2
- MF:C7H7FO2S
- Structure:
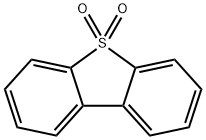
- Chemical Name:DIBENZOTHIOPHENE SULFONE
- CAS:1016-05-3
- MF:C12H8O2S
- Structure:
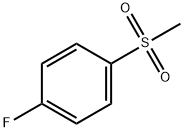
- Chemical Name:4-FLUOROPHENYL METHYL SULFONE
- CAS:455-15-2
- MF:C7H7FO2S
- Structure:
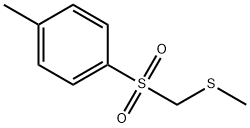
- Chemical Name:METHYLTHIOMETHYL P-TOLYL SULFONE
- CAS:59662-65-6
- MF:C9H12O2S2
- Structure:
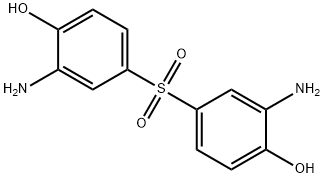
- Chemical Name:3,3'-Diamino-4,4'-dihydroxydiphenyl sulfone
- CAS:7545-50-8
- MF:C12H12N2O4S
- Structure:
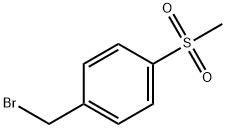
- Chemical Name:1-(BROMOMETHYL)-4-(METHYLSULFONYL)BENZENE
- CAS:53606-06-7
- MF:C8H9BrO2S
- Structure:
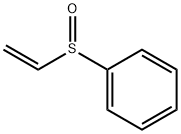
- Chemical Name:PHENYL VINYL SULFOXIDE
- CAS:20451-53-0
- MF:C8H8OS
- Structure:
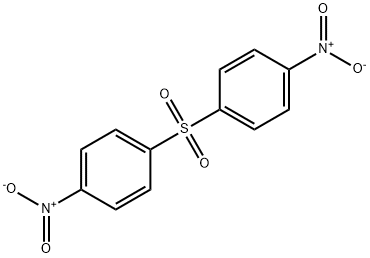
- Chemical Name:BIS(4-NITROPHENYL) SULFONE
- CAS:1156-50-9
- MF:C12H8N2O6S
- Structure:
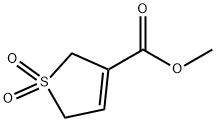
- Chemical Name:3-METHOXYCARBONYL-3-SULFOLENE
- CAS:67488-50-0
- MF:C6H8O4S
- Structure:
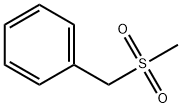
- Chemical Name:BENZYL METHYL SULFONE
- CAS:3112-90-1
- MF:C8H10O2S
- Structure:
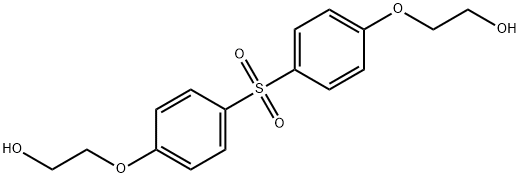
- Chemical Name:BIS[4-(2-HYDROXYETHOXY)PHENYL] SULFONE
- CAS:27205-03-4
- MF:C16H18O6S
- Structure:
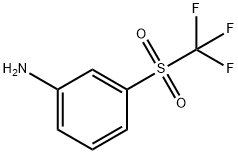
- Chemical Name:3-(TRIFLUOROMETHYLSULFONYL)ANILINE
- CAS:426-59-5
- MF:C7H6F3NO2S
- Structure:
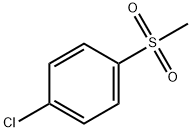
- Chemical Name:4-Methylsulfuryl chlorobenzene
- CAS:98-57-7
- MF:C7H7ClO2S
- Structure:
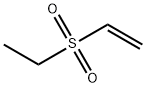
- Chemical Name:Ethyl vinyl sulfone
- CAS:1889-59-4
- MF:C4H8O2S
- Structure:
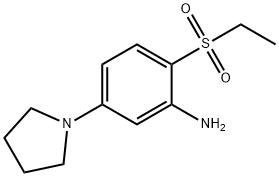
- Chemical Name:2-(Ethylsulfonyl)-5-(1-pyrrolidinyl)aniline
- CAS:1219972-54-9
- MF:C12H18N2O2S
- Structure:
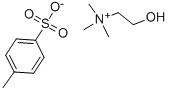
- Chemical Name:Choline tosylate
- CAS:55357-38-5
- MF:C12H21NO4S
- Structure:
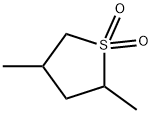
- Chemical Name:2,4-DIMETHYLSULFOLANE
- CAS:1003-78-7
- MF:C6H12O2S
- Structure:
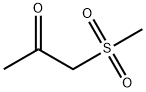
- Chemical Name:METHYLSULFONYLACETONE
- CAS:5000-46-4
- MF:C4H8O3S
- Structure:
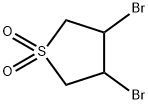
- Chemical Name:3,4-DIBROMOSULFOLANE
- CAS:15091-30-2
- MF:C4H6Br2O2S
- Structure:
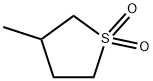
- Chemical Name:3-METHYLSULFOLANE
- CAS:872-93-5
- MF:C5H10O2S
- Structure:
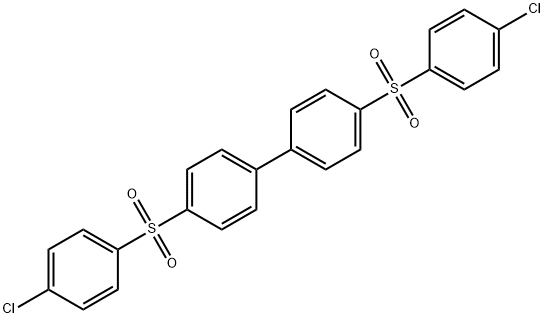
- Chemical Name:4,4'-Bis(4-chlorophenyl)sulfonyl-1,1'-biphenyl
- CAS:22287-56-5
- MF:C24H16Cl2O4S2
- Structure:
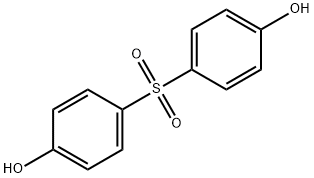
- Chemical Name:Bis(4-hydroxyphenyl) Sulfone
- CAS:80-09-1
- MF:C12H10O4S
- Structure:
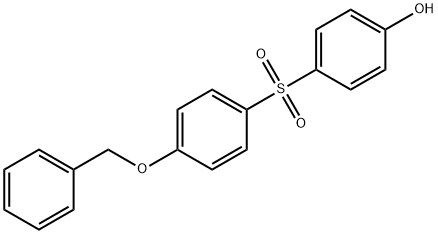
- Chemical Name:4-Benzyloxyphenyl 4-Hydroxyphenyl Sulfone
- CAS:63134-33-8
- MF:C19H16O4S
- Structure:

- Chemical Name:4-FLUORONAPHTHALENE-1-SULFONYL CHLORIDE
- CAS:316-69-8
- MF:C10H6ClFO2S
- Structure:
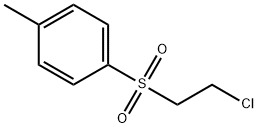
- Chemical Name:2-CHLOROETHYL P-TOLYL SULFONE
- CAS:22381-53-9
- MF:C9H11ClO2S
- Structure:
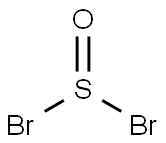
- Chemical Name:Thionyl bromide
- CAS:507-16-4
- MF:Br2OS
- Structure:
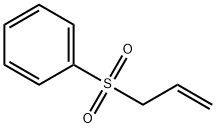
- Chemical Name:Allyl phenyl sulfone
- CAS:16212-05-8
- MF:C9H10O2S
- Structure:
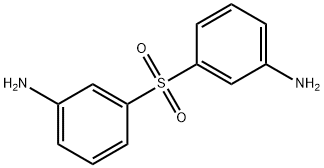
- Chemical Name:3,3'-Sulfonyldianiline
- CAS:599-61-1
- MF:C12H12N2O2S
- Structure:
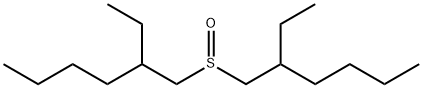
- Chemical Name:BIS(2-ETHYLHEXYL) SULFOXIDE
- CAS:82374-34-3
- MF:C16H34OS
- Structure:
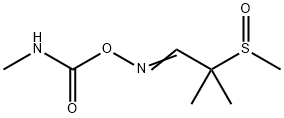
- Chemical Name:ALDICARB-SULFOXIDE
- CAS:1646-87-3
- MF:C7H14N2O3S
- Structure:
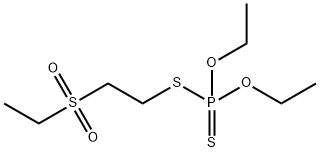
- Chemical Name:DISULFOTON-SULFONE
- CAS:2497-06-5
- MF:C8H19O4PS3
- Structure:
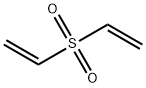
- Chemical Name:Divinyl sulphone
- CAS:77-77-0
- MF:C4H6O2S
- Structure:
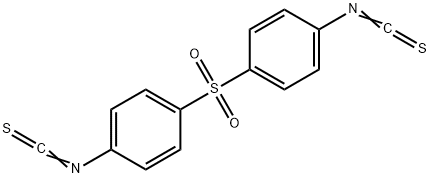
- Chemical Name:ISOTHIOCYANATOPHENYL SULFONE
- CAS:4430-49-3
- MF:C14H8N2O2S3
- Structure:
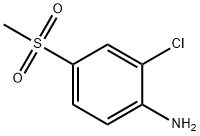
- Chemical Name:2-CHLORO-4-(METHYLSULFONYL)ANILINE
- CAS:13244-35-4
- MF:C7H8ClNO2S
- Structure:
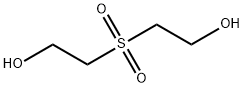
- Chemical Name:2,2'-SULFONYLDIETHANOL
- CAS:2580-77-0
- MF:C4H10O4S
- Structure:
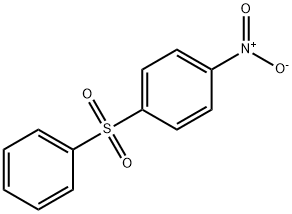
- Chemical Name:4-NITRODIPHENYL SULFONE
- CAS:1146-39-0
- MF:C12H9NO4S
- Structure:
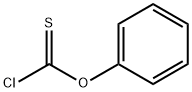
- Chemical Name:Phenyl chlorothionocarbonate
- CAS:1005-56-7
- MF:C7H5ClOS
- Structure:
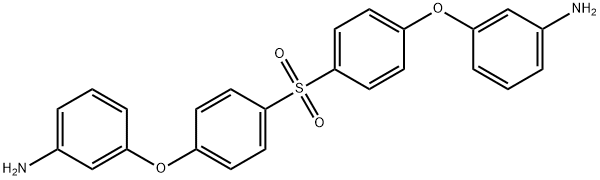
- Chemical Name:4,4'-BIS(3-AMINOPHENOXY)DIPHENYL SULFONE
- CAS:30203-11-3
- MF:C24H20N2O4S
- Structure:
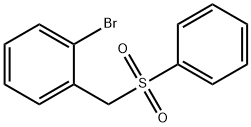
- Chemical Name:1-(BENZENESULFONYLMETHYL)-2-BROMOBENZENE
- CAS:92022-50-9
- MF:C13H11BrO2S
- Structure:
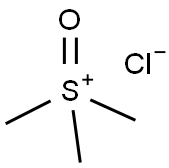
- Chemical Name:TRIMETHYLSULFOXONIUM CHLORIDE
- CAS:5034-06-0
- MF:C3H9ClOS
- Structure:
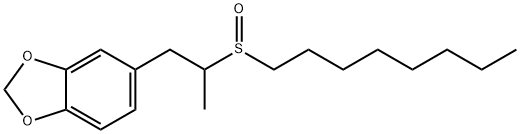
- Chemical Name:SULFOXIDE
- CAS:120-62-7
- MF:C18H28O3S
- Structure:
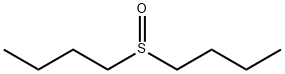
- Chemical Name:DI-N-BUTYL SULFOXIDE
- CAS:2168-93-6
- MF:C8H18OS
- Structure:
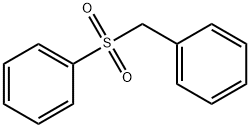
- Chemical Name:BENZYL PHENYL SULFONE
- CAS:3112-88-7
- MF:C13H12O2S
- Structure:
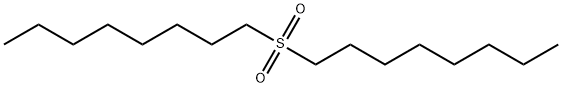
- Chemical Name:DI-N-OCTYL SULFONE
- CAS:7726-20-7
- MF:C16H34O2S
- Structure:
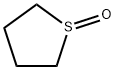
- Chemical Name:Tetramethylene sulfoxide
- CAS:1600-44-8
- MF:C4H8OS
- Structure:
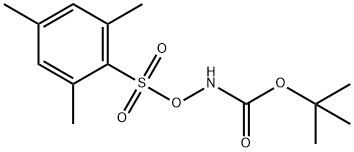
- Chemical Name:tert-Butyl (Mesitylsulfonyl)oxycarbaMate
- CAS:36016-39-4
- MF:C14H21NO5S
- Structure:
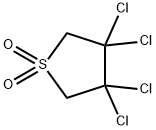
- Chemical Name:3,3,4,4-Tetrachlorotetrahydrothiophene 1,1-dioxide
- CAS:3737-41-5
- MF:C4H4Cl4O2S
- Structure:
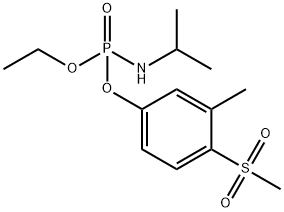
- Chemical Name:FENAMIPHOS SULFONE
- CAS:31972-44-8
- MF:C13H22NO5PS
- Structure:
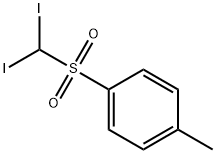
- Chemical Name:Tolyl diiodomethyl sulfone
- CAS:20018-09-1
- MF:C8H8I2O2S
- Structure:
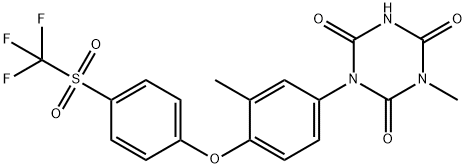
- Chemical Name:Ponazuril
- CAS:69004-04-2
- MF:C18H14F3N3O6S
- Structure:
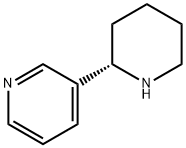
- Chemical Name:(-)-ANABASINE
- CAS:494-52-0
- MF:C10H14N2
- Structure:
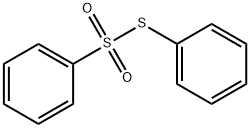
- Chemical Name:BENZENETHIOSULFONIC ACID S-PHENYL ESTER
- CAS:1212-08-4
- MF:C12H10O2S2
- Structure:
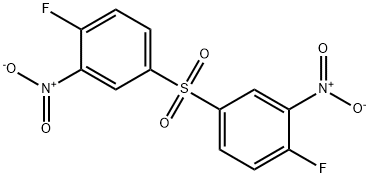
- Chemical Name:BIS(4-FLUORO-3-NITROPHENYL) SULFONE
- CAS:312-30-1
- MF:C12H6F2N2O6S
- Structure:
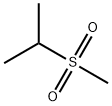
- Chemical Name:ISOPROPYL METHYL SULFONE
- CAS:4853-74-1
- MF:C4H10O2S
- Structure:
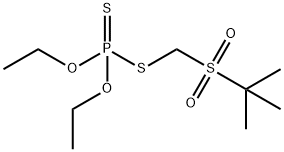
- Chemical Name:TERBUFOS-SULFONE
- CAS:56070-16-7
- MF:C9H21O4PS3
- Structure:
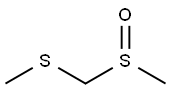
- Chemical Name:MMTS
- CAS:33577-16-1
- MF:C3H8OS2
- Structure:
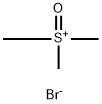
- Chemical Name:TRIMETHYLSULFOXONIUM BROMIDE
- CAS:25596-24-1
- MF:C3H9BrOS
- Structure:

- Chemical Name:DI-N-DODECYL SULFOXIDE
- CAS:2168-96-9
- MF:C24H50OS
- Structure:

- Chemical Name:2-CHLOROETHYL 4-CHLOROPHENYL SULFONE
- CAS:16191-84-7
- MF:C8H8Cl2O2S
- Structure:
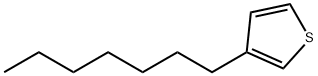
- Chemical Name:3-N-HEPTYLTHIOPHENE
- CAS:65016-61-7
- MF:C11H18S
- Structure:
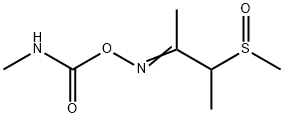
- Chemical Name:BUTOCARBOXIM SULFOXIDE
- CAS:34681-24-8
- MF:C7H14N2O3S
- Structure:
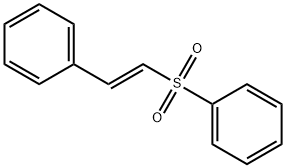
- Chemical Name:PHENYL TRANS-STYRYL SULFONE
- CAS:16212-06-9
- MF:C14H12O2S
- Structure:
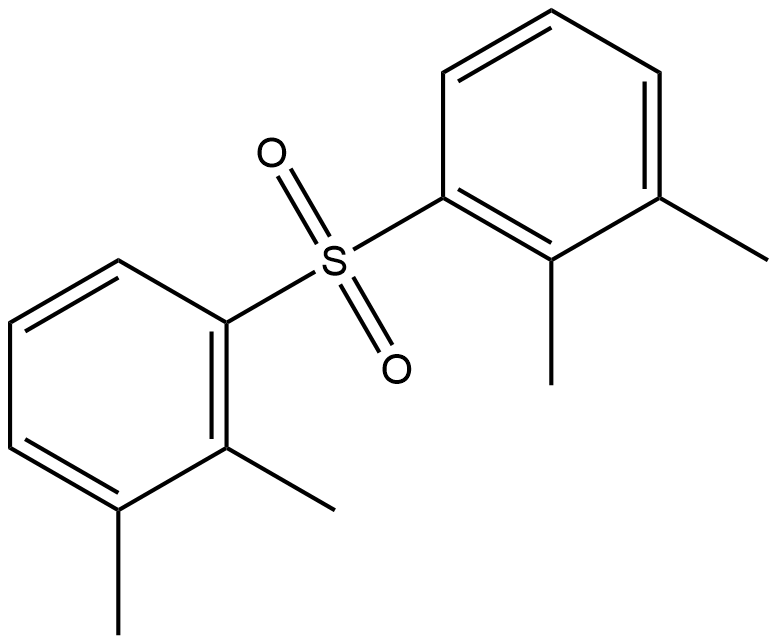
- Chemical Name:DIXYLYL SULFONE
- CAS:27043-27-2
- MF:C16H18O2S
- Structure:
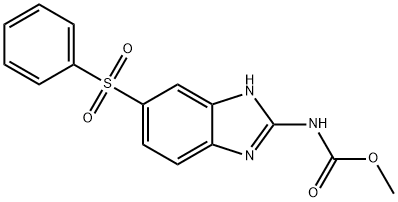
- Chemical Name:Fenbendazole sulfone
- CAS:54029-20-8
- MF:C15H13N3O4S
- Structure:
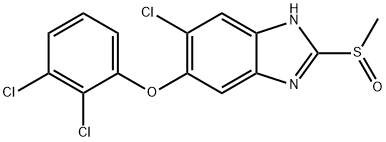
- Chemical Name:Triclabendazole sulfoxide
- CAS:100648-13-3
- MF:C14H9Cl3N2O2S
- Structure:
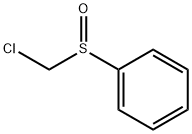
- Chemical Name:CHLOROMETHYL PHENYL SULFOXIDE
- CAS:7205-94-9
- MF:C7H7ClOS
- Structure:
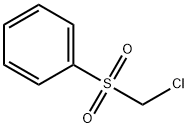
- Chemical Name:CHLOROMETHYL PHENYL SULFONE
- CAS:7205-98-3
- MF:C7H7ClO2S
- Structure:
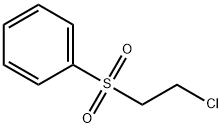
- Chemical Name:2-CHLOROETHYL PHENYL SULFONE
- CAS:938-09-0
- MF:C8H9ClO2S
- Structure:
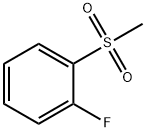
- Chemical Name:2-FLUOROPHENYLMETHYLSULFONE
- CAS:654-47-7
- MF:C7H7FO2S
- Structure:
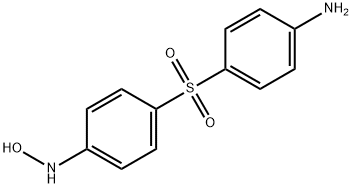
- Chemical Name:DAPSONE HYDROXYLAMINE
- CAS:32695-27-5
- MF:C12H12N2O3S
- Structure:
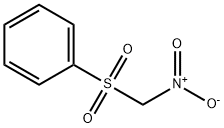
- Chemical Name:NITROMETHYL PHENYL SULFONE
- CAS:21272-85-5
- MF:C7H7NO4S
- Structure:
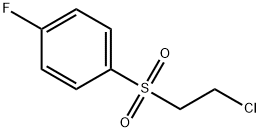
- Chemical Name:2-CHLOROETHYL 4-FLUOROPHENYL SULFONE
- CAS:33330-46-0
- MF:C8H8ClFO2S
- Structure:
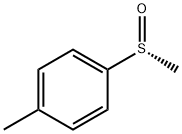
- Chemical Name:(R)-(+)-Methyl p-tolyl sulfoxide
- CAS:1519-39-7
- MF:C8H10OS
- Structure:
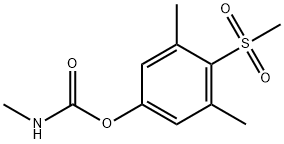
- Chemical Name:METHIOCARB SULFONE
- CAS:2179-25-1
- MF:C11H15NO4S
- Structure:
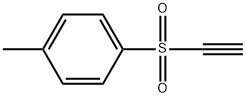
- Chemical Name:ETHYNYL P-TOLYL SULFONE
- CAS:13894-21-8
- MF:C9H8O2S
- Structure:
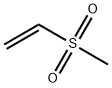
- Chemical Name:Methyl vinyl sulfone
- CAS:3680-02-2
- MF:C3H6O2S
- Structure:
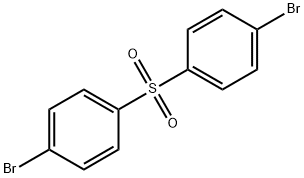
- Chemical Name:4,4'-DIBROMO DIPHENYL SULFONE
- CAS:2050-48-8
- MF:C12H8Br2O2S
- Structure:
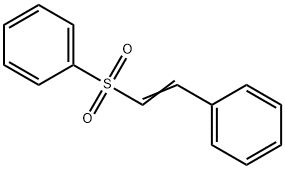
- Chemical Name:PHENYL TRANS-STYRYL SULFONE 99
- CAS:5418-11-1
- MF:C14H12O2S
- Structure:
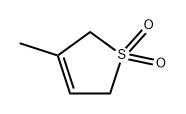
- Chemical Name:3-METHYL-2,5-DIHYDROTHIOPHENE-1,1-DIOXIDE
- CAS:1193-10-8
- MF:C5H8O2S
- Structure:
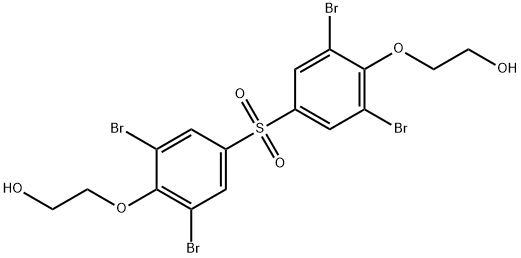
- Chemical Name:BIS[4-(2-HYDROXYETHOXY)-3,5-DIBROMOPHENYL] SULFONE
- CAS:53714-39-9
- MF:C16H14Br4O6S
- Structure:
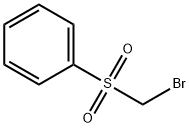
- Chemical Name:BROMOMETHYL PHENYL SULFONE
- CAS:19169-90-5
- MF:C7H7BrO2S
- Structure:
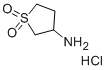
- Chemical Name:3-AMINOTETRAHYDRO-1H-1LAMBDA6-THIOPHENE-1,1-DIONE HYDROCHLORIDE
- CAS:51642-03-6
- MF:C4H10ClNO2S
- Structure:
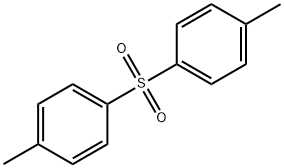
- Chemical Name:DI-P-TOLYL SULFONE
- CAS:599-66-6
- MF:C14H14O2S
- Structure:
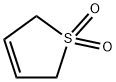
- Chemical Name:3-SULFOLENE
- CAS:77-79-2
- MF:C4H6O2S
- Structure:
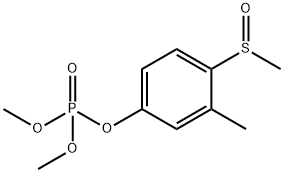
- Chemical Name:FENTHION-OXON-SULFOXIDE
- CAS:6552-13-2
- MF:C10H15O5PS
- Structure:
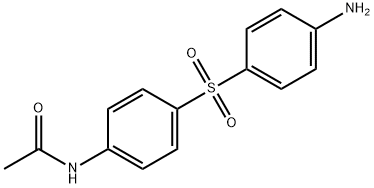
- Chemical Name:N-MONOACETYL-4,4'-DIAMINODIPHENYL SULFONE
- CAS:565-20-8
- MF:C14H14N2O3S
- Structure:
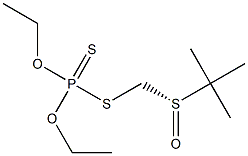
- Chemical Name:TERBUFOS-SULFOXIDE
- CAS:10548-10-4
- MF:C9H21O3PS3
- Structure:
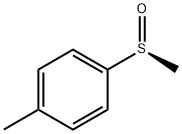
- Chemical Name:(S)-(-)-METHYL P-TOLYL SULFOXIDE
- CAS:5056-07-5
- MF:C8H10OS
- Structure:
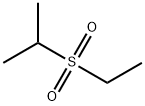
- Chemical Name:ETHYL ISOPROPYL SULFONE
- CAS:4853-75-2
- MF:C5H12O2S
- Structure:
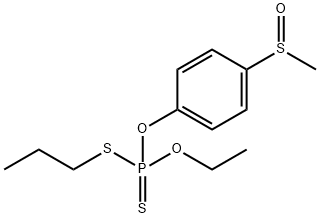
- Chemical Name:SULPROFOS-SULFOXIDE
- CAS:34643-47-5
- MF:C12H19O3PS3
- Structure:
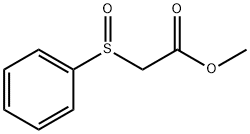
- Chemical Name:METHYL (PHENYLSULFINYL)ACETATE
- CAS:14090-83-6
- MF:C9H10O3S
- Structure:
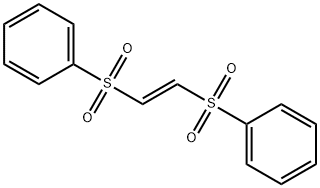
- Chemical Name:TRANS-1,2-BIS(PHENYLSULFONYL)ETHYLENE
- CAS:963-16-6
- MF:C14H12O4S2
- Structure:
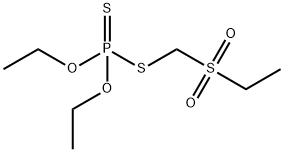
- Chemical Name:PHORATE SULFONE
- CAS:2588-04-7
- MF:C7H17O4PS3
- Structure:
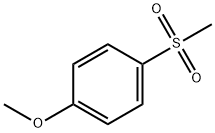
- Chemical Name:4-METHOXYPHENYLMETHYLSULFONE
- CAS:3517-90-6
- MF:C8H10O3S
- Structure:
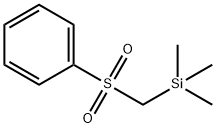
- Chemical Name:TRIMETHYLSILYLBENZENESULFONATE
- CAS:17872-92-3
- MF:C10H16O2SSi