Halogenated aliphatic hydrocarbons
The halogenated aliphatic hydrocarbon is a derivative in which the hydrogen atom in the open chain hydrocarbon is replaced by a halogen element, i.e., a fluoro, chloro, bromo or iodo derivative.
According to the hydrocarbon structure it can be divided into saturated halogenated hydrocarbons (halogenated alkanes) and unsaturated halogenated hydrocarbons (such as halogenated olefins).
According to the number of halogen atoms contained in the molecule it is divided into a halogenated compounds, dihalides and polyhalides.
The physical and chemical properties of the derivatives vary greatly. At room temperature, the general lower halogenated hydrocarbons (C1 ~ C4) is a gas, higher halogenated hydrocarbons (> C16) is a solid, the middle of the two is liquid. In halohydrocarbon molecules, if the hydrocarbon is same, then the proportion and boiling point of chlorinated hydrocarbons are the lowest, while the iodinated hydrocarbons are highest. If the halogen is same , the boiling point increases with the increase of molecular weight. The vast majority of halogenated hydrocarbons are insoluble in water, and soluble in organic solvents. Halogen is a functional group of halogenated hydrocarbons whose chemical activity is mainly determined by the halogen atom. Generally speaking, carbon-halogen bond is more active than carbon-carbon bond or carbon-hydrogen bond , can be broken easily, gets the nature of a nucleophile. In addition, the activity of halogen atoms relates with the structure of group greatly which is directly connected with the halogen atoms. Haloalkanes are fairly active compounds, while some halogen in halogenated olefins are very active, some are not active, their activity depends on the location of halogen and double bond. In terms of biological activity, brominated and iodinated hydrocarbons are more toxic than chlorinated hydrocarbons; fluorinated hydrocarbons are chemically stable and less toxic, with some exceptions.
Halogenated hydrocarbons commonly used in industry are mostly volatile liquid. Most halogenated hydrocarbons are not flammable and explosive, commonly used as solvents, chemical raw materials or intermediates, and widely used in agriculture, medicine and light industry.
- Structure:

- Chemical Name:1,8-Dibromooctane
- CAS:4549-32-0
- MF:C8H16Br2
- Structure:
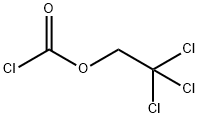
- Chemical Name:2,2,2-Trichloroethyl chloroformate
- CAS:17341-93-4
- MF:C3H2Cl4O2
- Structure:
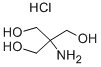
- Chemical Name:TRIS hydrochloride
- CAS:1185-53-1
- MF:C4H12ClNO3
- Structure:
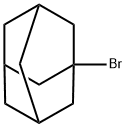
- Chemical Name:1-Bromoadamantane
- CAS:768-90-1
- MF:C10H15Br
- Structure:
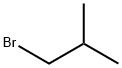
- Chemical Name:1-Bromo-2-methylpropane
- CAS:78-77-3
- MF:C4H9Br
- Structure:
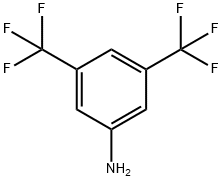
- Chemical Name:3,5-Bis(trifluoromethyl)aniline
- CAS:328-74-5
- MF:C8H5F6N
- Structure:

- Chemical Name:9-Bromo-1-nonanol
- CAS:55362-80-6
- MF:C9H19BrO
- Structure:
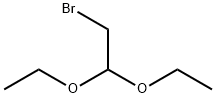
- Chemical Name:Bromoacetaldehyde diethyl acetal
- CAS:2032-35-1
- MF:C6H13BrO2
- Structure:
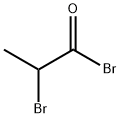
- Chemical Name:2-Bromopropionyl bromide
- CAS:563-76-8
- MF:C3H4Br2O
- Structure:
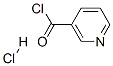
- Chemical Name:Nicotinoyl chloride hydrochloride
- CAS:20260-53-1
- MF:C6H5Cl2NO
- Structure:
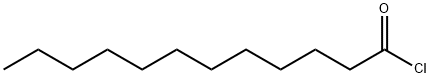
- Chemical Name:Lauroyl chloride
- CAS:112-16-3
- MF:C12H23ClO
- Structure:
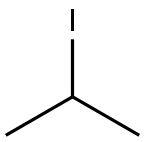
- Chemical Name:2-Iodopropane
- CAS:75-30-9
- MF:C3H7I
- Structure:
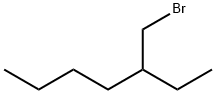
- Chemical Name:2-Ethylhexyl bromide
- CAS:18908-66-2
- MF:C8H17Br
- Structure:

- Chemical Name:Diiodomethane
- CAS:75-11-6
- MF:CH2I2
- Structure:
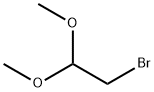
- Chemical Name:Bromoacetaldehyde dimethyl acetal
- CAS:7252-83-7
- MF:C4H9BrO2
- Structure:
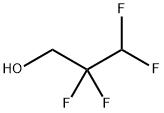
- Chemical Name:Tetrafluoro-1-propanol
- CAS:76-37-9
- MF:C3H4F4O
- Structure:
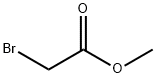
- Chemical Name:Methyl bromoacetate
- CAS:96-32-2
- MF:C3H5BrO2
- Structure:
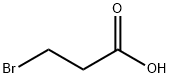
- Chemical Name:3-Bromopropionic acid
- CAS:590-92-1
- MF:C3H5BrO2
- Structure:
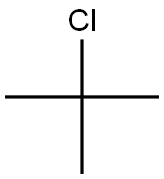
- Chemical Name:2-Chloro-2-methylpropane
- CAS:507-20-0
- MF:C4H9Cl
- Structure:

- Chemical Name:1-Chlorohexadecane
- CAS:4860-03-1
- MF:C16H33Cl
- Structure:

- Chemical Name:1-Chlorohexane
- CAS:544-10-5
- MF:C6H13Cl
- Structure:

- Chemical Name:1,6-Dichlorohexane
- CAS:2163-00-0
- MF:C6H12Cl2
- Structure:
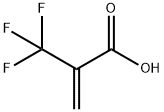
- Chemical Name:2-(Trifluoromethyl)acrylic acid
- CAS:381-98-6
- MF:C4H3F3O2
- Structure:
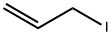
- Chemical Name:ALLYL IODIDE
- CAS:556-56-9
- MF:C3H5I
- Structure:

- Chemical Name:trans-1,3-Dichloropropene
- CAS:10061-02-6
- MF:C3H4Cl2
- Structure:
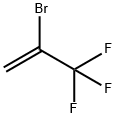
- Chemical Name:2-BROMO-3,3,3-TRIFLUOROPROPENE
- CAS:1514-82-5
- MF:C3H2BrF3
- Structure:

- Chemical Name:1,2-Bis(2-chloroethoxy)ethane
- CAS:112-26-5
- MF:C6H12Cl2O2
- Structure:
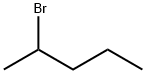
- Chemical Name:2-Bromopentane
- CAS:107-81-3
- MF:C5H11Br
- Structure:
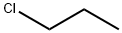
- Chemical Name:1-Chloropropane
- CAS:540-54-5
- MF:C3H7Cl
- Structure:
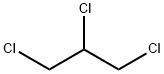
- Chemical Name:1,2,3-Trichloropropane
- CAS:96-18-4
- MF:C3H5Cl3
- Structure:
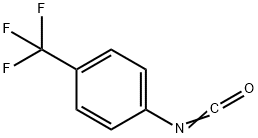
- Chemical Name:4-(TRIFLUOROMETHYL)PHENYL ISOCYANATE
- CAS:1548-13-6
- MF:C8H4F3NO
- Structure:
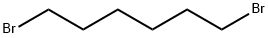
- Chemical Name:1,6-Dibromohexane
- CAS:629-03-8
- MF:C6H12Br2
- Structure:
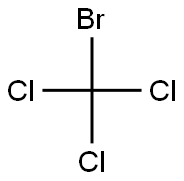
- Chemical Name:Bromotrichloromethane
- CAS:75-62-7
- MF:CBrCl3
- Structure:
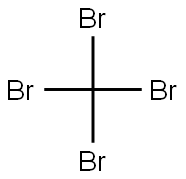
- Chemical Name:Carbon tetrabromide
- CAS:558-13-4
- MF:CBr4
- Structure:
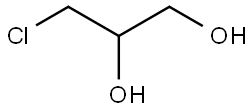
- Chemical Name:3-Chloro-1,2-propanediol
- CAS:96-24-2
- MF:C3H7ClO2
- Structure:
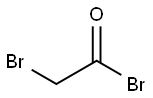
- Chemical Name:Bromoacetyl bromide
- CAS:598-21-0
- MF:C2H2Br2O
- Structure:

- Chemical Name:1,9-DIBROMONONANE
- CAS:4549-33-1
- MF:C9H18Br2
- Structure:
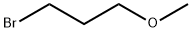
- Chemical Name:1-Bromo-3-methoxypropane
- CAS:36865-41-5
- MF:C4H9BrO
- Structure:
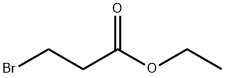
- Chemical Name:Ethyl 3-bromopropionate
- CAS:539-74-2
- MF:C5H9BrO2
- Structure:
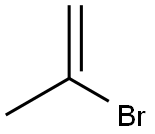
- Chemical Name:2-BROMOPROPENE
- CAS:557-93-7
- MF:C3H5Br
- Structure:
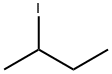
- Chemical Name:2-Iodobutane
- CAS:513-48-4
- MF:C4H9I
- Structure:

- Chemical Name:1-Chlorooctane
- CAS:111-85-3
- MF:C8H17Cl
- Structure:
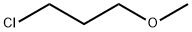
- Chemical Name:3-Chloropropyl methyl ether
- CAS:36215-07-3
- MF:C4H9ClO
- Structure:
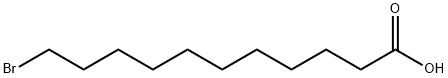
- Chemical Name:11-Bromoundecanoic acid
- CAS:2834-05-1
- MF:C11H21BrO2
- Structure:
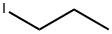
- Chemical Name:1-Iodopropane
- CAS:107-08-4
- MF:C3H7I
- Structure:
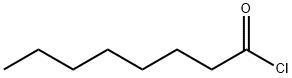
- Chemical Name:Octanoyl chloride
- CAS:111-64-8
- MF:C8H15ClO
- Structure:
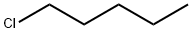
- Chemical Name:1-Chloropentane
- CAS:543-59-9
- MF:C5H11Cl
- Structure:
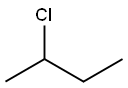
- Chemical Name:2-Chlorobutane
- CAS:78-86-4
- MF:C4H9Cl
- Structure:

- Chemical Name:2-Methoxyethyl chloride
- CAS:627-42-9
- MF:C3H7ClO
- Structure:
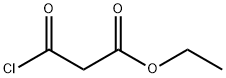
- Chemical Name:Ethyl malonyl chloride
- CAS:36239-09-5
- MF:C5H7ClO3
- Structure:
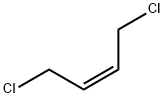
- Chemical Name:cis-1,4-Dichloro-2-butene
- CAS:1476-11-5
- MF:C4H6Cl2
- Structure:
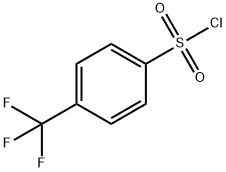
- Chemical Name:4-(Trifluoromethyl)benzene-1-sulfonyl chloride
- CAS:2991-42-6
- MF:C7H4ClF3O2S
- Structure:

- Chemical Name:trans-1,4-Dichloro-2-butene
- CAS:110-57-6
- MF:C4H6Cl2
- Structure:
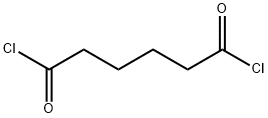
- Chemical Name:Adipoyl chloride
- CAS:111-50-2
- MF:C6H8Cl2O2
- Structure:
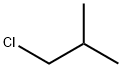
- Chemical Name:1-Chloro-2-methylpropane
- CAS:513-36-0
- MF:C4H9Cl
- Structure:
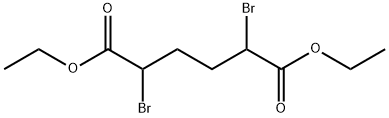
- Chemical Name:Diethyl 2,5-dibromohexanedioate
- CAS:869-10-3
- MF:C10H16Br2O4
- Structure:
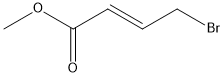
- Chemical Name:Methyl 4-bromocrotonate
- CAS:1117-71-1
- MF:C5H7BrO2
- Structure:
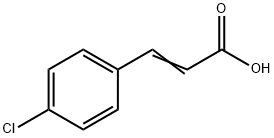
- Chemical Name:4-Chlorocinnamic acid
- CAS:1615-02-7
- MF:C9H7ClO2
- Structure:
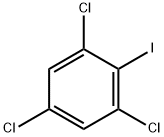
- Chemical Name:2,4,6-Trichloroiodobenzene
- CAS:6324-50-1
- MF:C6H2Cl3I
- Structure:
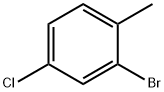
- Chemical Name:2-Bromo-4-chlorotoluene
- CAS:27139-97-5
- MF:C7H6BrCl
- Structure:
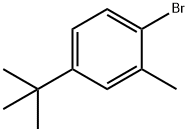
- Chemical Name:Benzene, 1-bromo-4-(1,1-dimethylethyl)-2-methyl-
- CAS:854637-01-7
- MF:C11H15Br
- Structure:
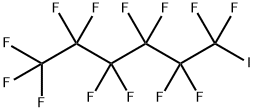
- Chemical Name:Perfluoro-1-iodohexane
- CAS:355-43-1
- MF:C6F13I
- Structure:
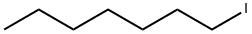
- Chemical Name:1-Iodoheptane
- CAS:4282-40-0
- MF:C7H15I
- Structure:

- Chemical Name:1-Chlorododecane
- CAS:112-52-7
- MF:C12H25Cl
- Structure:
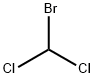
- Chemical Name:BROMODICHLOROMETHANE
- CAS:75-27-4
- MF:CHBrCl2
- Structure:

- Chemical Name:3-FLUOROPROPAN-1-OL
- CAS:462-43-1
- MF:C3H7FO
- Structure:
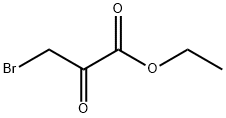
- Chemical Name:Ethyl bromopyruvate
- CAS:70-23-5
- MF:C5H7BrO3
- Structure:
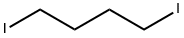
- Chemical Name:1,4-Diiodobutane
- CAS:628-21-7
- MF:C4H8I2
- Structure:
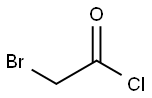
- Chemical Name:Bromoacetyl chloride
- CAS:22118-09-8
- MF:C2H2BrClO
- Structure:

- Chemical Name:1-Iodohexane
- CAS:638-45-9
- MF:C6H13I
- Structure:

- Chemical Name:5-Chloropentanol
- CAS:5259-98-3
- MF:C5H11ClO
- Structure:
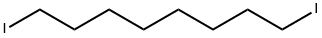
- Chemical Name:1,8-DIIODOOCTANE
- CAS:24772-63-2
- MF:C8H16I2
- Structure:
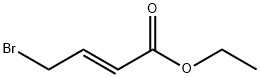
- Chemical Name:Ethyl 4-bromocrotonate
- CAS:37746-78-4
- MF:C6H9BrO2
- Structure:

- Chemical Name:1-Chlorodecane
- CAS:1002-69-3
- MF:C10H21Cl
- Structure:

- Chemical Name:1-IODODECANE
- CAS:2050-77-3
- MF:C10H21I
- Structure:

- Chemical Name:1-IODOHEXADECANE
- CAS:544-77-4
- MF:C16H33I
- Structure:
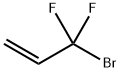
- Chemical Name:3-Bromo-3,3-difluoropropene
- CAS:420-90-6
- MF:C3H3BrF2
- Structure:
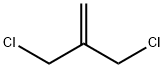
- Chemical Name:2-(CHLOROMETHYL)ALLYLTRICHLOROSILANE
- CAS:1871-57-4
- MF:C4H6Cl2
- Structure:
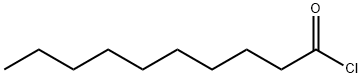
- Chemical Name:Decanoyl chloride
- CAS:112-13-0
- MF:C10H19ClO
- Structure:
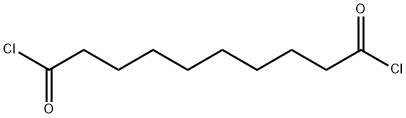
- Chemical Name:Sebacoyl chloride
- CAS:111-19-3
- MF:C10H16Cl2O2
- Structure:
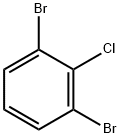
- Chemical Name:1,3-dibromo-2-chlorobenzene
- CAS:19230-27-4
- MF:C6H3Br2Cl
- Structure:
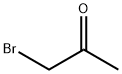
- Chemical Name:BROMOACETONE
- CAS:598-31-2
- MF:C3H5BrO
- Structure:
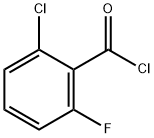
- Chemical Name:2-Chloro-6-fluorobenzene-1-carbonyl chloride
- CAS:79455-63-3
- MF:C7H3Cl2FO
- Structure:
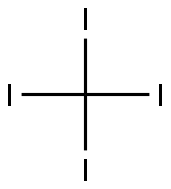
- Chemical Name:CARBON TETRAIODIDE
- CAS:507-25-5
- MF:CI4
- Structure:
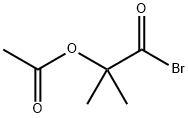
- Chemical Name:2-Acetoxy-2-methylpropionyl bromide
- CAS:40635-67-4
- MF:C6H9BrO3
- Structure:
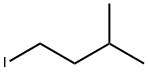
- Chemical Name:1-Iodo-3-methylbutane
- CAS:541-28-6
- MF:C5H11I
- Structure:
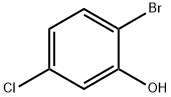
- Chemical Name:2-Bromo-5-chlorophenol
- CAS:13659-23-9
- MF:C6H4BrClO
- Structure:
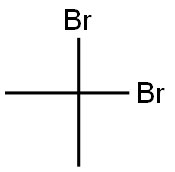
- Chemical Name:2,2-Dibromopropane
- CAS:594-16-1
- MF:C3H6Br2
- Structure:
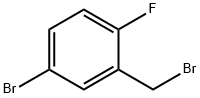
- Chemical Name:2-Fluoro-5-bromobenzyl bromide
- CAS:99725-12-9
- MF:C7H5Br2F
- Structure:
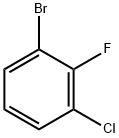
- Chemical Name:1-BROMO-3-CHLORO-2-FLUOROBENZENE
- CAS:144584-65-6
- MF:C6H3BrClF
- Structure:

- Chemical Name:7-Bromo-1-heptanol
- CAS:10160-24-4
- MF:C7H15BrO
- Structure:
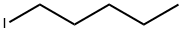
- Chemical Name:1-Iodopentane
- CAS:628-17-1
- MF:C5H11I
- Structure:
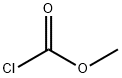
- Chemical Name:Methyl chloroformate
- CAS:79-22-1
- MF:C2H3ClO2
- Chemical Name:Chloroalkanes C10-13
- CAS:85535-84-8
- MF:
- Structure:
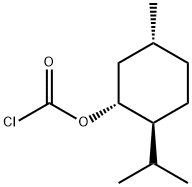
- Chemical Name:(-)-MENTHYL CHLOROFORMATE
- CAS:14602-86-9
- MF:C11H19ClO2
- Structure:
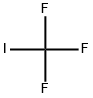
- Chemical Name:Trifluoromethyl iodide
- CAS:2314-97-8
- MF:CF3I
- Structure:
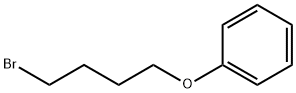
- Chemical Name:4-Phenoxybutyl bromide
- CAS:1200-03-9
- MF:C10H13BrO
- Structure:
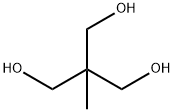
- Chemical Name:1,1,1-Tris(hydroxymethyl)ethane
- CAS:77-85-0
- MF:C5H12O3
- Structure:
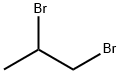
- Chemical Name:1,2-Dibromopropane
- CAS:78-75-1
- MF:C3H6Br2
- Structure:
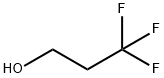
- Chemical Name:3,3,3-TRIFLUORO-1-PROPANOL
- CAS:2240-88-2
- MF:C3H5F3O