Phosphines
Asymmetric phosphine compounds and their applications as well as the emergence of a variety of novel compounds have greatly enriched the subjects of organic phosphorus chemistry. Phosphorus-containing antioxidants, stabilizers and the extractant, complexing agent of rare elements can be widely applied to the chemical industry. Organic phosphine compound in vivo is an important kind of energy and an important component for the formation of the biopolymer DNA and RNA backbone.
The organic phosphorus compound has a wide application in nucleic acids, coenzymes, organophosphorus nerve gases, organophosphorus insecticides, organophosphorus fungicides, organophosphorus herbicides, chemotherapeutic agents, plasticizers, antioxidants, surfactants, complexing agents, extraction agent of organic phosphorus, flotation agent and a flame retardant and so on.
The chemical structure of the organic phosphorus compound can be roughly divided into the following categories:
1. Phosphonate. Phosphoric acid is a tribasic acid, i.e., three of which may be replaced by hydrogen atoms with these hydrogen atoms being replaced by an organic group, known as phosphonate. These three groups, when being not exactly the same, are known as mixed esters. In organophosphorous pesticides, there are a lot of mixed phosphate esters, such as dichlorvos that is a mixture of phosphoric acid esters.
2. Mono-, di- and tri-phosphonothiolate. The oxygen atom of the phosphate molecule can be replaced by a sulfur atom, known as thiosulfate phosphonate, such as parathion, dimethoate, kitazine that all belonging to such structures.
3. Phosphoramidites and Thioxophosphamide. When the hydroxyl group in the phosphoric acid is replaced by an amino group, it is called phosphoramidites. The remaining oxygen atom in the phosphoramidites molecule may be replaced by a sulfur atom, and become thioxophosphamide such as methamidophos and crufomate.
4. Pyrophosphate, thiopyrophosphoric acid and pyrophosphate amide. Two phosphate molecules get rid of one molecule of water to form pyrophosphate with the hydrogen, oxygen and hydroxyl groups being able to be respectively, replaced by sulfur atom and amino group such as sulfotep.
5. Thio-phosphonate and phosphonate. One hydroxyl group is replaced by organic group which formed P-C bond inside the molecule, namely phosphonate. There have been some kinds of phosphonate and corresponding sulfur-substituted compounds as pesticides promotion, such as benzene parathion.
6. Phosphorous acid derivative. Some pesticides are derivatives of phosphorous acid. In the molecule of phosphorous acid, the phosphorus is trivalent such as deleaf defolian.
7. Phosphine. When the hydrogen inside the phosphine molecules is replaced by organic group, it is called phosphine. The structure of these compounds is similar with that of nitrogen compounds such as ammonia, amines and ammonium salts, known as orthopedic phosphine and phosphine.
8. Phosphoric acid or the hydroxy group inside phosphoric acid is replaced by a halogen.
- Structure:
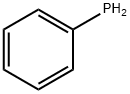
- Chemical Name:Phenyl phosphine
- CAS:638-21-1
- MF:C6H7P
- Structure:
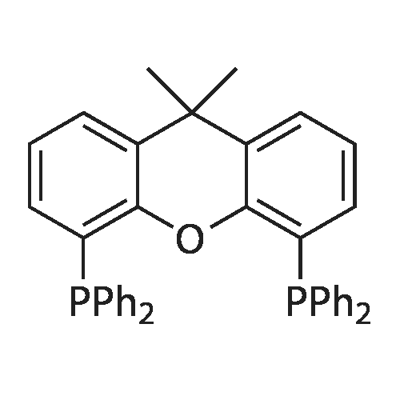
- Chemical Name:4,5-Bis(diphenylphosphino)-9,9-dimethylxanthene
- CAS:161265-03-8
- MF:C39H32OP2
- Structure:
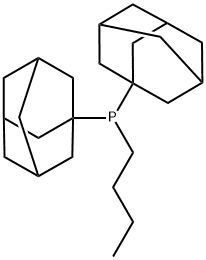
- Chemical Name:Butyldi-1-adamantylphosphine
- CAS:321921-71-5
- MF:C24H39P
- Structure:
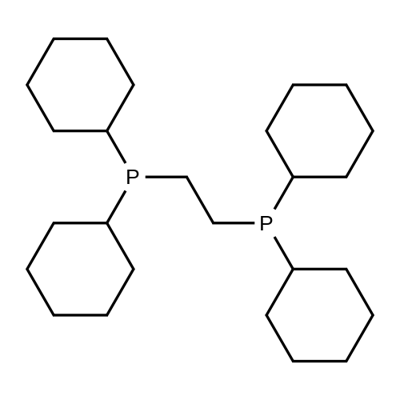
- Chemical Name:1,2-BIS(DICYCLOHEXYLPHOSPHINO)ETHANE
- CAS:23743-26-2
- MF:C26H48P2
- Structure:
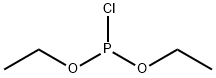
- Chemical Name:DIETHYL CHLOROPHOSPHITE
- CAS:589-57-1
- MF:C4H10ClO2P
- Structure:
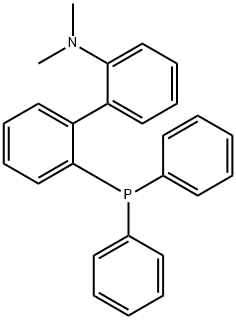
- Chemical Name:2-Diphenylphosphino-2'-(N,N-dimethylamino)biphenyl
- CAS:240417-00-9
- MF:C26H24NP
- Structure:
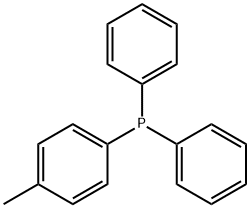
- Chemical Name:DIPHENYL(P-TOLYL)PHOSPHINE
- CAS:1031-93-2
- MF:C19H17P
- Structure:
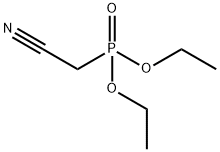
- Chemical Name:Diethyl cyanomethylphosphonate
- CAS:2537-48-6
- MF:C6H12NO3P
- Structure:
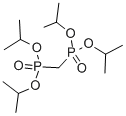
- Chemical Name:Tetraisopropyl methylenediphosphonate
- CAS:1660-95-3
- MF:C13H30O6P2
- Structure:
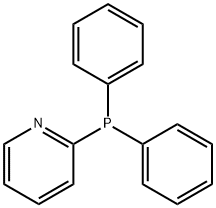
- Chemical Name:Diphenyl-2-pyridylphosphine
- CAS:37943-90-1
- MF:C17H14NP
- Structure:
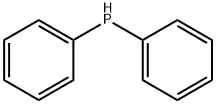
- Chemical Name:Diphenylphosphine
- CAS:829-85-6
- MF:C12H11P
- Structure:
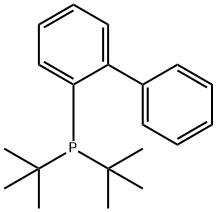
- Chemical Name:2-(Di-tert-butylphosphino)biphenyl
- CAS:224311-51-7
- MF:C20H27P
- Structure:
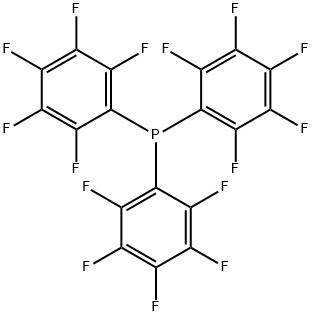
- Chemical Name:TRIS(PENTAFLUOROPHENYL)PHOSPHINE
- CAS:1259-35-4
- MF:C18F15P
- Structure:
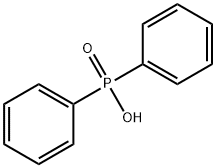
- Chemical Name:Diphenylphosphinic acid
- CAS:1707-03-5
- MF:C12H11O2P
- Structure:
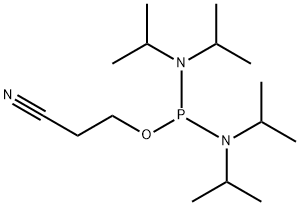
- Chemical Name:2-Cyanoethyl N,N,N',N'-tetraisopropylphosphorodiamidite
- CAS:102691-36-1
- MF:C15H32N3OP
- Structure:
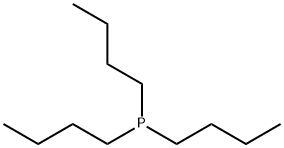
- Chemical Name:Tributylphosphine
- CAS:998-40-3
- MF:C12H27P
- Structure:
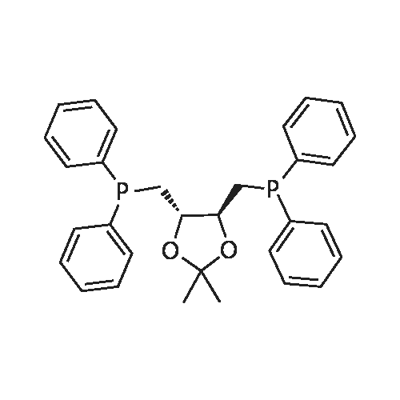
- Chemical Name:(+)-DIOP
- CAS:37002-48-5
- MF:C31H32O2P2
- Structure:
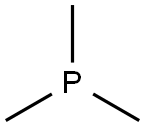
- Chemical Name:Trimethylphosphine
- CAS:594-09-2
- MF:C3H9P
- Structure:
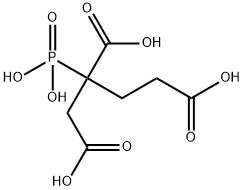
- Chemical Name:2-Phosphonobutane-1,2,4-tricarboxylic acid
- CAS:37971-36-1
- MF:C7H11O9P
- Structure:
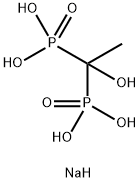
- Chemical Name:(1-Hydroxyethylidene)bis-phosphonic acid tetrasodium salt
- CAS:3794-83-0
- MF:C2H9NaO7P2
- Structure:
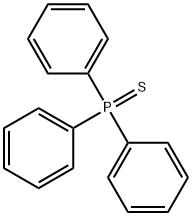
- Chemical Name:TRIPHENYLPHOSPHINE SULFIDE
- CAS:3878-45-3
- MF:C18H15PS
- Structure:
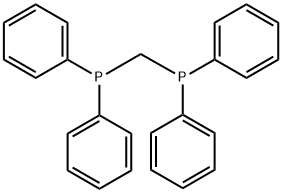
- Chemical Name:Bis(diphenylphosphino)methane
- CAS:2071-20-7
- MF:C25H22P2
- Structure:
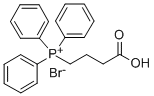
- Chemical Name:(3-CARBOXYPROPYL)TRIPHENYLPHOSPHONIUM BROMIDE
- CAS:17857-14-6
- MF:C22H22BrO2P
- Structure:
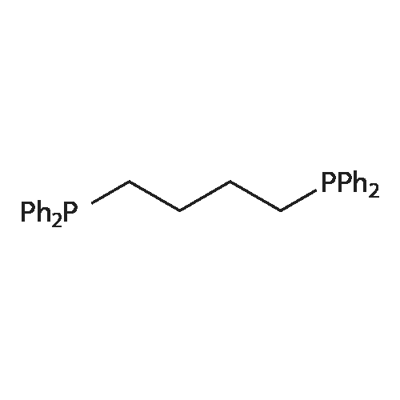
- Chemical Name:1,4-Bis(diphenylphosphino)butane
- CAS:7688-25-7
- MF:C28H28P2
- Structure:
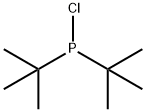
- Chemical Name:Di-tert-butylchlorophosphane
- CAS:13716-10-4
- MF:C8H18ClP
- Structure:
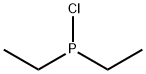
- Chemical Name:CHLORO(DIETHYL)PHOSPHINE
- CAS:686-69-1
- MF:C4H10ClP
- Structure:
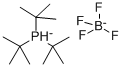
- Chemical Name:Tri-tert-butylphosphine tetrafluoroborate
- CAS:131274-22-1
- MF:C12H28P.BF4
- Structure:
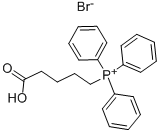
- Chemical Name:(4-Carboxybutyl)triphenylphosphonium bromide
- CAS:17814-85-6
- MF:C23H24BrO2P
- Structure:
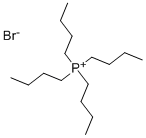
- Chemical Name:Tetrabutylphosphonium bromide
- CAS:3115-68-2
- MF:C16H36BrP
- Structure:
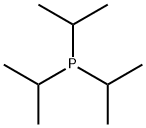
- Chemical Name:TRIISOPROPYLPHOSPHINE
- CAS:6476-36-4
- MF:C9H21P
- Structure:
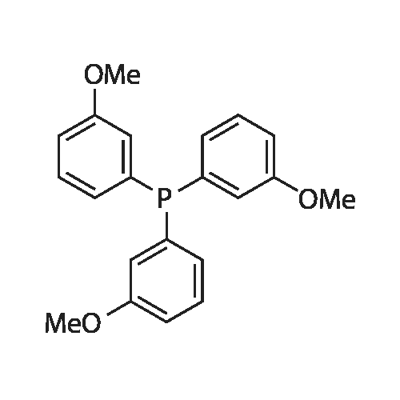
- Chemical Name:Tris(3-methoxyphenyl)phosphine
- CAS:29949-84-6
- MF:C21H21O3P
- Structure:
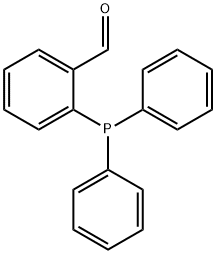
- Chemical Name:2-Diphenylphosphinobenzaldehyde
- CAS:50777-76-9
- MF:C19H15OP
- Structure:
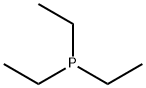
- Chemical Name:Triethylphosphine
- CAS:554-70-1
- MF:C6H15P
- Structure:
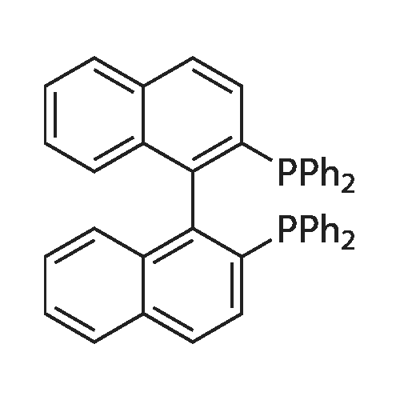
- Chemical Name:1.1'-Binaphthyl-2.2'-diphemyl phosphine
- CAS:98327-87-8
- MF:C44H32P2
- Structure:
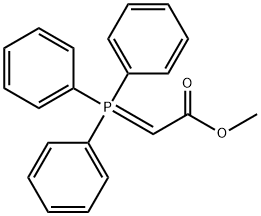
- Chemical Name:Methyl (triphenylphosphoranylidene)acetate
- CAS:2605-67-6
- MF:C21H19O2P
- Structure:
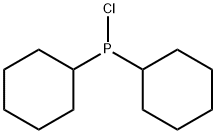
- Chemical Name:Dicyclohexylchlorophosphine
- CAS:16523-54-9
- MF:C12H22ClP
- Structure:
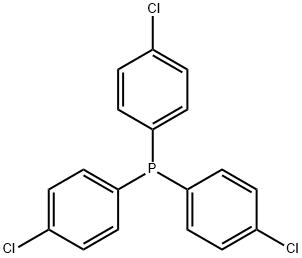
- Chemical Name:TRIS(4-CHLOROPHENYL)PHOSPHINE
- CAS:1159-54-2
- MF:C18H12Cl3P
- Structure:
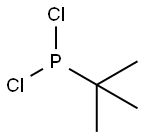
- Chemical Name:tert-Butyldichlorophosphine
- CAS:25979-07-1
- MF:C4H9Cl2P
- Structure:
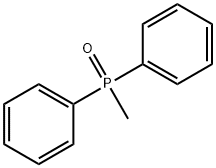
- Chemical Name:METHYLDIPHENYLPHOSPHINE OXIDE
- CAS:2129-89-7
- MF:C13H13OP
- Structure:
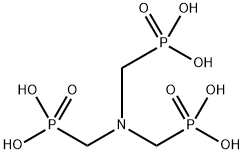
- Chemical Name:Amino tris(methylene phosphonic acid)
- CAS:6419-19-8
- MF:C3H12NO9P3
- Structure:
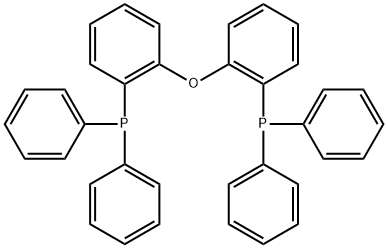
- Chemical Name:(OXYDI-2,1-PHENYLENE)BIS(DIPHENYLPHOSPHINE)
- CAS:166330-10-5
- MF:C36H28OP2
- Structure:
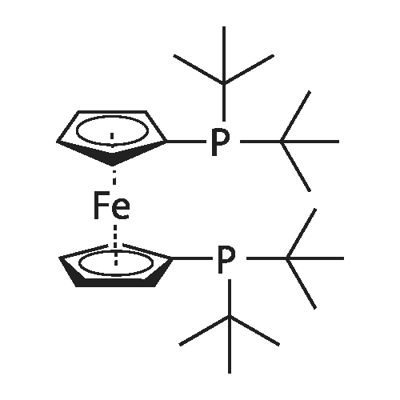
- Chemical Name:1,1'-Bis(di-tert-butylphosphino)ferrocene
- CAS:84680-95-5
- MF:C26H44FeP210*
- Structure:
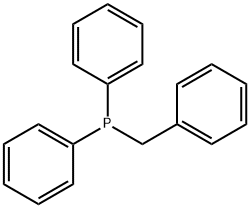
- Chemical Name:Benzyldiphenylphosphine
- CAS:7650-91-1
- MF:C19H17P
- Structure:
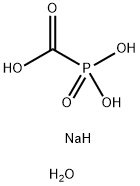
- Chemical Name:Phosphonoformic acid trisodium salt hexahydrate
- CAS:34156-56-4
- MF:CH6NaO6P
- Structure:
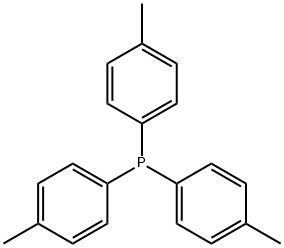
- Chemical Name:TRI-P-TOLYLPHOSPHINE
- CAS:1038-95-5
- MF:C21H21P
- Structure:
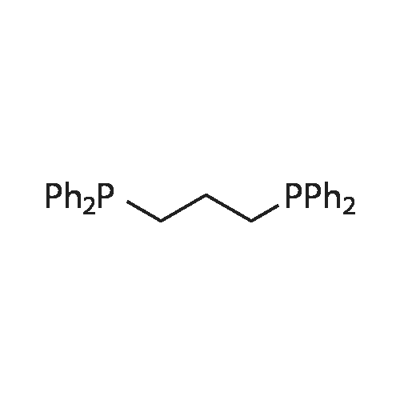
- Chemical Name:1,3-Bis(diphenylphosphino)propane
- CAS:6737-42-4
- MF:C27H26P2
- Structure:
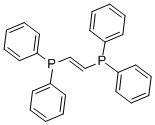
- Chemical Name:TRANS-1,2-BIS(DIPHENYLPHOSPHINO)ETHYLENE
- CAS:983-81-3
- MF:C26H22P2
- Structure:
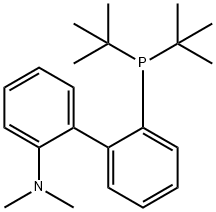
- Chemical Name:2-Di-t-butylphosphino-2'-(N,N-dimethylamino)biphenyl
- CAS:224311-49-3
- MF:C22H32NP
- Structure:
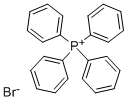
- Chemical Name:Tetraphenylphosphonium bromide
- CAS:2751-90-8
- MF:C24H20BrP
- Structure:
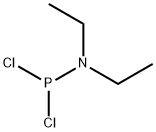
- Chemical Name:DIETHYLPHOSPHORAMIDOUS DICHLORIDE
- CAS:1069-08-5
- MF:C4H10Cl2NP
- Structure:
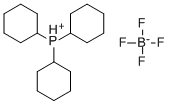
- Chemical Name:TRICYCLOHEXYLPHOSPHONIUM TETRAFLUOROBORATE
- CAS:58656-04-5
- MF:C18H34BF4P
- Structure:
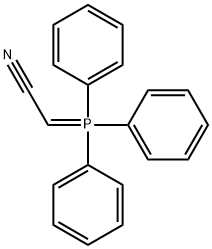
- Chemical Name:(Triphenylphosphoranylidene)acetonitrile
- CAS:16640-68-9
- MF:C20H16NP
- Structure:
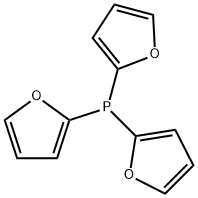
- Chemical Name:TRI(2-FURYL)PHOSPHINE
- CAS:5518-52-5
- MF:C12H9O3P
- Structure:
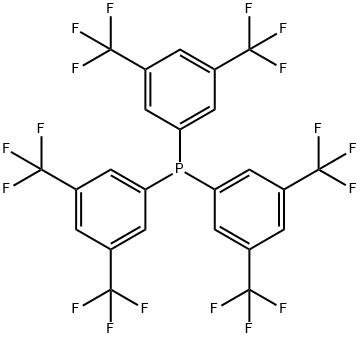
- Chemical Name:TRIS[3,5-BIS(TRIFLUOROMETHYL)PHENYL]PHOSPHINE
- CAS:175136-62-6
- MF:C24H9F18P
- Structure:
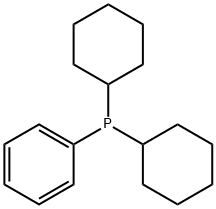
- Chemical Name:Dicyclohexylphenylphosphine
- CAS:6476-37-5
- MF:C18H27P
- Structure:
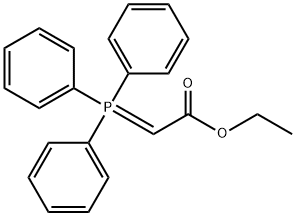
- Chemical Name:Ethyl (triphenylphosphoranylidene)acetate
- CAS:1099-45-2
- MF:C22H21O2P
- Structure:
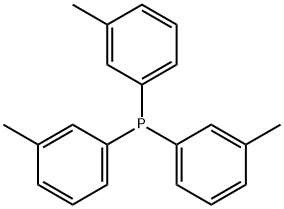
- Chemical Name:TRI-M-TOLYLPHOSPHINE
- CAS:6224-63-1
- MF:C21H21P
- Structure:
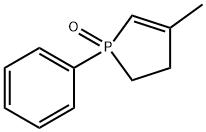
- Chemical Name:3-Methyl-1-phenyl-2-phospholene 1-oxide
- CAS:707-61-9
- MF:C11H13OP
- Structure:
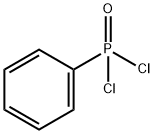
- Chemical Name:Phenylphosphonic dichloride
- CAS:824-72-6
- MF:C6H5Cl2OP
- Structure:
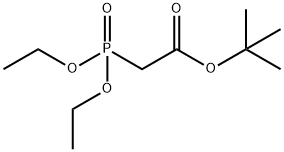
- Chemical Name:tert-Butyl diethylphosphonoacetate
- CAS:27784-76-5
- MF:C10H21O5P
- Structure:
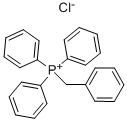
- Chemical Name:Benzyltriphenylphosphonium chloride
- CAS:1100-88-5
- MF:C25H22ClP
- Structure:
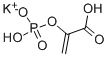
- Chemical Name:PHOSPHOENOLPYRUVIC ACID MONOPOTASSIUM SALT
- CAS:4265-07-0
- MF:C3H4KO6P
- Structure:
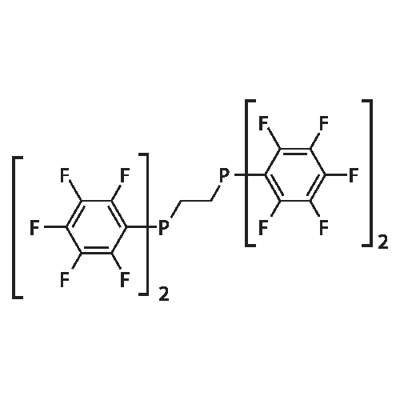
- Chemical Name:1,2-BIS(DIPENTAFLUOROPHENYLPHOSPHINO)ETHANE
- CAS:76858-94-1
- MF:C26H4F20P2
- Structure:
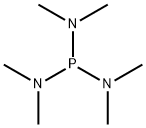
- Chemical Name:Hexamethylphosphorous triamide
- CAS:1608-26-0
- MF:C6H18N3P
- Structure:
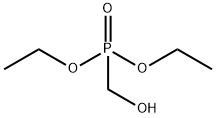
- Chemical Name:Diethyl (hydroxymethyl)phosphonate
- CAS:3084-40-0
- MF:C5H13O4P
- Structure:
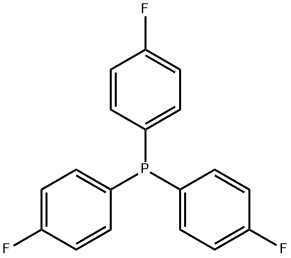
- Chemical Name:Tris(4-fluorophenyl)phosphine
- CAS:18437-78-0
- MF:C18H12F3P
- Structure:
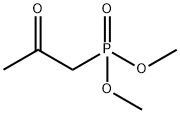
- Chemical Name:Dimethyl acetylmethylphosphonate
- CAS:4202-14-6
- MF:C5H11O4P
- Structure:
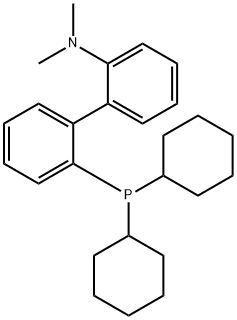
- Chemical Name:2-Dicyclohexylphosphino-2'-(N,N-dimethylamino)biphenyl
- CAS:213697-53-1
- MF:C26H36NP
- Structure:
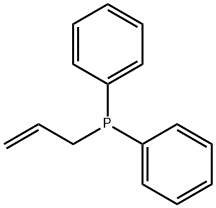
- Chemical Name:ALLYLDIPHENYLPHOSPHINE
- CAS:2741-38-0
- MF:C15H15P
- Structure:
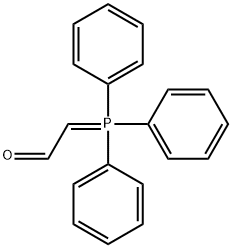
- Chemical Name:(FORMYLMETHYLENE)TRIPHENYLPHOSPHORANE
- CAS:2136-75-6
- MF:C20H17OP
- Structure:
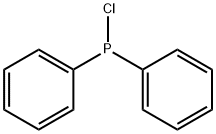
- Chemical Name:Chlorodiphenylphosphine
- CAS:1079-66-9
- MF:C12H10ClP
- Structure:
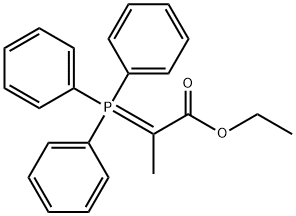
- Chemical Name:Ethyl 2-(triphenylphosphoranylidene)propionate
- CAS:5717-37-3
- MF:C23H23O2P
- Structure:
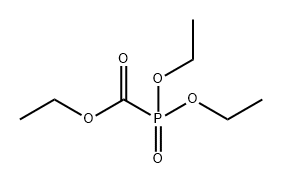
- Chemical Name:Ethyl diethoxyphosphinylformate
- CAS:1474-78-8
- MF:C7H15O5P
- Structure:
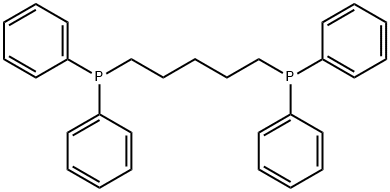
- Chemical Name:1,5-Bis(diphenylphosphino)pentane
- CAS:27721-02-4
- MF:C29H30P2
- Structure:
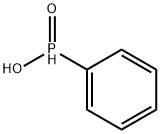
- Chemical Name:Phenylphosphinic acid
- CAS:1779-48-2
- MF:C6H7O2P
- Structure:
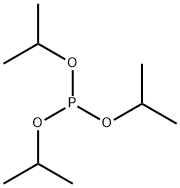
- Chemical Name:Triisopropyl phosphite
- CAS:116-17-6
- MF:C9H21O3P
- Structure:
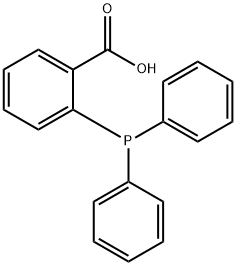
- Chemical Name:2-(Diphenylphosphino)benzoic acid
- CAS:17261-28-8
- MF:C19H15O2P
- Structure:
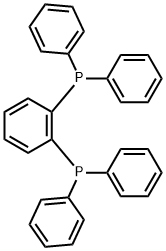
- Chemical Name:1,2-BIS(DIPHENYLPHOSPHINO)BENZENE
- CAS:13991-08-7
- MF:C30H24P2
- Structure:
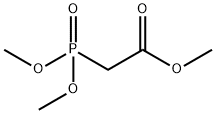
- Chemical Name:Trimethyl phosphonoacetate
- CAS:5927-18-4
- MF:C5H11O5P
- Structure:
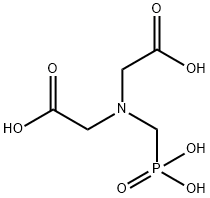
- Chemical Name:N-(Carboxymethyl)-N-(phosphonomethyl)-glycine
- CAS:5994-61-6
- MF:C5H10NO7P
- Structure:
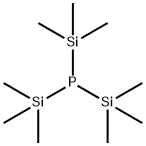
- Chemical Name:TRIS(TRIMETHYLSILYL)PHOSPHINE
- CAS:15573-38-3
- MF:C9H27PSi3
- Structure:
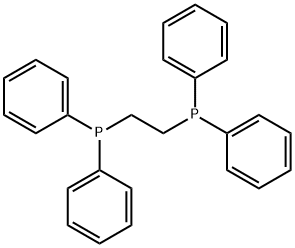
- Chemical Name:1,2-Bis(diphenylphosphino)ethane
- CAS:1663-45-2
- MF:C26H24P2
- Structure:
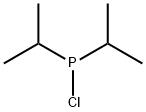
- Chemical Name:Chlorodiisopropylphosphine
- CAS:40244-90-4
- MF:C6H14ClP
- Structure:
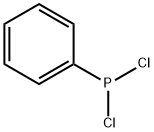
- Chemical Name:Dichlorophenylphosphine
- CAS:644-97-3
- MF:C6H5Cl2P
- Structure:
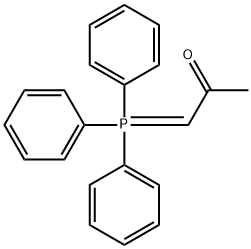
- Chemical Name:(ACETYLMETHYLENE)TRIPHENYLPHOSPHORANE
- CAS:1439-36-7
- MF:C21H19OP
- Structure:
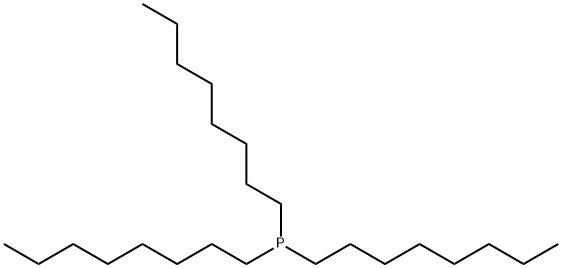
- Chemical Name:Trioctylphosphine
- CAS:4731-53-7
- MF:C24H51P
- Structure:
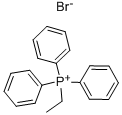
- Chemical Name:Ethyltriphenylphosphonium bromide
- CAS:1530-32-1
- MF:C20H20BrP
- Structure:
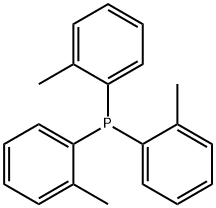
- Chemical Name:Tri(o-tolyl)phosphine
- CAS:6163-58-2
- MF:C21H21P
- Structure:
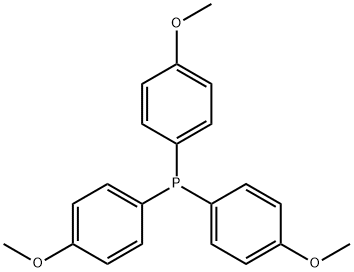
- Chemical Name:Tris(4-methoxyphenyl)phosphine
- CAS:855-38-9
- MF:C21H21O3P
- Structure:
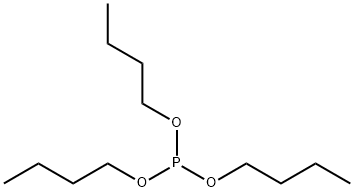
- Chemical Name:TRIBUTYL PHOSPHITE
- CAS:102-85-2
- MF:C12H27O3P
- Structure:
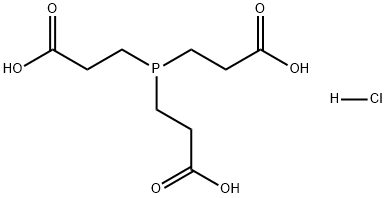
- Chemical Name:Tris(2-carboxyethyl)phosphine hydrochloride
- CAS:51805-45-9
- MF:C9H15O6P.ClH
- Structure:
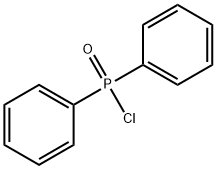
- Chemical Name:Diphenylphosphinic Chloride
- CAS:1499-21-4
- MF:C12H10ClOP
- Structure:
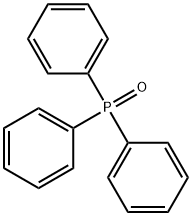
- Chemical Name:Triphenylphosphine oxide
- CAS:791-28-6
- MF:C18H15OP
- Structure:
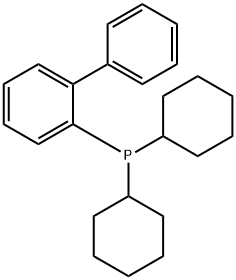
- Chemical Name:2-(Dicyclohexylphosphino)biphenyl
- CAS:247940-06-3
- MF:C24H31P
- Structure:
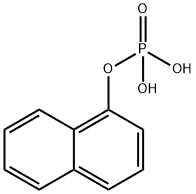
- Chemical Name:1-NAPHTHYL PHOSPHATE
- CAS:1136-89-6
- MF:C10H9O4P
- Structure:
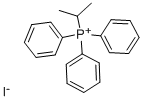
- Chemical Name:ISOPROPYLTRIPHENYLPHOSPHONIUM IODIDE
- CAS:24470-78-8
- MF:C21H22IP
- Structure:
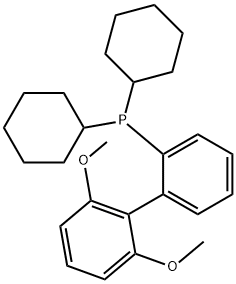
- Chemical Name:2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl
- CAS:657408-07-6
- MF:C26H35O2P
- Structure:
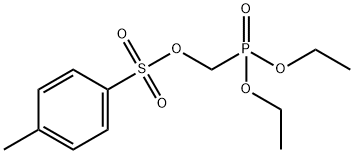
- Chemical Name:Diethyl (tosyloxy)methylphosphonate
- CAS:31618-90-3
- MF:C12H19O6PS
- Structure:
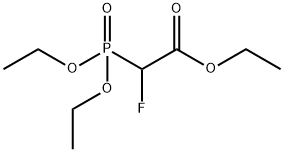
- Chemical Name:Triethyl 2-fluoro-2-phosphonoacetate
- CAS:2356-16-3
- MF:C8H16FO5P
- Structure:
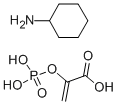
- Chemical Name:Phosphoenolpyruvic acid cyclohexylammonium salt
- CAS:10526-80-4
- MF:C6H13N.C3H5O6P