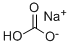
Sodium Hydrocarbonate can often be used as a safer alternative to sodium hydroxide, and as such can be used as a wash to remove any acidic impurities from a "crude" liquid, producing a purer sample.
Reaction of Sodium Hydrocarbonate and an acid produces a salt and carbonic acid, which readily decomposes to carbon dioxide and water: NaHCO3 + HCl → NaCl + H2O+CO2
H2CO3 → H2O + CO2(g)
Sodium Hydrocarbonate reacts with acetic acid (found in vinegar), producing sodium acetate, water, and carbon dioxide:
NaHCO3 + CH3COOH → CH3COONa + H2O + CO2(g)