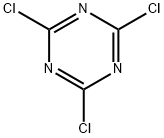
Chemical Properties
Sodium Hydrocarbonate is slowly decomposes to CO2 and Na2CO3 in aqueous solution at ambient temperature; decomposes to Na2CO3 in boiling water; aqueous solution slightly alkaline; pH of 0.1M solution at 25°C is about 8.3; insoluble in alcohol; decomposes in acids.
Physical properties
Sodium Hydrocarbonate is a white water-soluble crystalline solid.It has an alkaline taste, loses carbon dioxide at 270°C (518 °F).
Uses
Sodium Hydrocarbonate, used in the formof baking soda and baking powder, is the most common leavening agent; used in the manufacture of many sodium salts; source of CO2; ingredient of baking powder, effervescent salts and beverages; in fire extinguishers, cleaning Compounds.
Production Methods
Sodium Hydrocarbonate is manufactured either by passing carbon dioxide into a cold saturated solution of sodium carbonate, or by the ammonia–soda (Solvay) process, in which first ammonia and then carbon dioxide is passed into a sodium chloride solution to precipitate Sodium Hydrocarbonate while the more soluble ammonium chloride remains in solution.
Brand name
Neut (Abbott); Soda Mint (Lilly)
Chemical Reactivity
Sodium Hydrocarbonate can often be used as a safer alternative to sodium hydroxide, and as such can be used as a wash to remove any acidic impurities from a "crude" liquid, producing a purer sample.
Reaction of Sodium Hydrocarbonate and an acid produces a salt and carbonic acid, which readily decomposes to carbon dioxide and water: NaHCO3 + HCl → NaCl + H2O+CO2
H2CO3 → H2O + CO2(g)
Sodium Hydrocarbonate reacts with acetic acid (found in vinegar), producing sodium acetate, water, and carbon dioxide:
NaHCO3 + CH3COOH → CH3COONa + H2O + CO2(g)
Pharmaceutical Applications
Sodium Hydrocarbonate is usually administered orally in order to regulate the serum pH. Imbalances of the plasma pH can be due to problems occurring in the kidneys such as renal tubular acidosis. Within the kidneys, blood is filtered before it passes through the tubular part of the nephrons where re-absorption or secretion of important salts and others takes place. In renal tubular acidosis, the kidneys either fail to filter or secrete acid ions (H+) from the plasma (secretion takes place in the distal tubule), or to recover bicarbonate ions (HCO3-) from the filtrate (passive re-absorption takes place in the proximal tubule, active re-absorption at the distal tubule), which is necessary to balance the pH. In the view of this mode of action, the pharmaceutically active component of Sodium Hydrocarbonate is the bicarbonate anion, but the cation Na+ is responsible for solubility and compatibility.
Clinical Use
Sodium Hydrocarbonate is indicated to treat metabolic acidosis and alkalinize the urine. It is also used as adjunctive therapy in treating hypercalcemic or hyperkalemia crises.