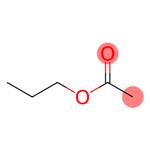
Description
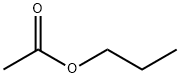
Propyl acetate, also known as propyl ethanoate, is an organic compound with a molecular formula of C5H10O2. It is a clear and colourless liquid with with a mild fruity odor. It is highly flammable with a flash point of 14°C and a flammability rating of 3. It is highly miscible with all common organic solvents (alcohols, ketones, glycols, esters) but has only slight miscibility in water. Propyl acetate is found in apple and formed by the esterification of acetic acid and 1-propanol (known as acondensation reaction), often via Fischer–Speier esterification, with sulfuric acid as a catalyst and water produced as a byproduct. It is primarily intended as a solvent in the coatings and printing inks industries. It is widely used in fragrances and as a flavor additive due to its odor. It also acts as a good solvent for cellulose nitrate, acrylates, alkyd resins, rosin, plasticizers, waxes, oils and fats.
Chemical Properties
Propyl acetate has a fruity (pear–raspberry) odor with a pleasant, bittersweet flavor reminiscent of pear on dilution. The Odor Threshold is 70 milligram per cubic meter and 2.8 milligram per cubic meter (New Jersey Fact Sheet).
Physical properties
Clear, colorless, flammable liquid with a pleasant, pear-like odor. Experimentally determined
detection and recognition odor threshold concentrations were 200 μg/m
3 (48 ppb
v) and 600 μg/m
3
(140 ppb
v), respectively (Hellman and Small, 1974). An odor threshold concentration of 240 ppb
v
was determined by a triangular odor bag method (Nagata and Takeuchi, 1990). Cometto-Mu?iz
and Cain (1991) reported an average nasal pungency threshold concentration of 17,575 ppm
v.
Occurrence
Reported found in apple, apple juice, apricot, banana, black currants, guava, grapes, melon, peach, pears, pineapple,
plum, strawberry, tomato, vinegar, wheat and rye bread, feta cheese, Gruyere cheese, domiati cheese, yogurt, beef fat, beer,
cognac, bourbon and malt whiskey, cider, grape wines, cocoa, potato chips, honey, passion fruit, starfruit, fig, prickly pear, jackfruit,
litchi, sake, loquat, mountain papaya, arrack, nectarine and pepino fruit.
Uses
n-Propyl acetate is used as a solvent for cellulose derivatives, plastics, and resins; in flavors and perfumes; and in organic synthesis.
Preparation
Propyl Acetate is formed by the esterification of acetic acid and 1-propanol with sulfuric acid as a catalyst and water produced as a byproduct. or By direct acetylation of propyl alcohol.
Production Methods
n-Propyl acetate is manufactured from acetic acid and a
mixture of propene and propane in the presence of a zinc
chloride catalyst. It is used as a solvent for nitrocellulose-
based lacquers, waxes, polyamide inks, acrylic inks, and
insecticide formulations .
Manufacturers include Eastman Chemical Company,
Hoechst Celanese Corporation, and Union Carbide
Corporation.
Definition
ChEBI: Propyl acetate is an acetate ester obtained by the formal condensation of acetic acid with propanol. It has a role as a fragrance and a plant metabolite. It derives from a propan-1-ol.
Aroma threshold values
Detection: 2.7 to 11 ppm. Aroma characteristics at 1.0%: pungent, solventlike ethereal, fruity lift, green
banana sweet with an apple and tropical fruit nuance.
Taste threshold values
Taste characteristics at 10 to 15 ppm: bubble gum estery, fruity, ethereal, tutti-frutti, banana and honey.
General Description
N-propyl acetate appears as a clear colorless liquid with a pleasant odor. Flash point 58°F. Less dense than water, Vapors are heavier than air.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Propyl acetate is an ester. Propyl acetate is colorless, highly flammable liquid, moderately toxic. Dangerous fire hazard when exposed to heat, flame, sparks, or strong oxidizers. When heated to decomposition Propyl acetate emits acrid smoke and irritating fumes [Lewis, 3rd ed., 1993, p. 1093].
Hazard
Flammable, dangerous fire risk, explosive
limits in air 2–8%. Eye and upper respiratory tract
irritant.
Health Hazard
The acute toxicity of n-propyl acetate islow in test animals. The toxicity, however,is slightly greater than ethyl acetate andisopropyl acetate. Exposure to its vaporsproduces irritation of the eyes, nose, andthroat and narcotic effects. A 5-hour expo sure to 9000- and 6000-ppm concentrationsproduced narcotic symptoms in cats andmice, respectively (Flury and Wirth 1933).A 4-hour exposure to 8000 ppm was lethalto rats. Ingestion of the liquid can cause narcotic action. A high dose can cause death. Adose of 3000 mg/kg by subcutaneous admin istration was lethal to cats. The liquid maycause mild irritation upon contact with skin
LD50 value, oral (mice): 8300 mg/kg.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Flammability and Explosibility
Highly flammable
Safety Profile
Moderately toxic by
intraperitoneal and subcutaneous routes.
Mildly toxic by ingestion and inhalation.
Human systemic effects by inhalation:
lachrymation, cough. A skin irritant. A
narcotic at high concentrations. Isopropyl
acetate is slightly less narcotic than normal propyl acetate. A flammable liquid and
dangerous fire hazard when exposed to heat,
flame, or oxidizers. Explosive in the form of
vapor when exposed to heat or flame. Can
react vigorously with oxidizing materials. To
fight fire, use alcohol foam, CO2, dry
chemical. When heated to decomposition it
emits acrid smoke and irritating fumes.
Potential Exposure
Propyl acetate is a used as a solvent
for plastics and cellulose ester resins; perfume ingredient;
component of food flavoring. It is also used as a chemical
intermediate.
Environmental Fate
Photolytic. Reported rate constants for the reaction of n-propyl acetate and OH radicals in the
atmosphere and aqueous solution are 2.7 x 10
-12 cm
3/molecule?sec (Hendry and Kenley, 1979) and
2.30 x 10
-13 cm
3/molecule?sec (Wallington et al., 1988b).
Chemical/Physical. Slowly hydrolyzes in water forming acetic acid and 1-propanol.
At an influent concentration of 1,000 mg/L, treatment with GAC resulted in an effluent
concentration of 248 mg/L. The adsorbability of the carbon used was 149 mg/g carbon (Guisti et
al., 1974).
Shipping
UN1276 n-Propyl acetate, Hazard Class: 3;
Labels: 3-Flammable liquid.
Purification Methods
Wash the ester with saturated aqueous NaHCO3 until neutral, then with saturated aqueous NaCl. Dry it with MgSO4 and fractionally distil it. [Beilstein 2 IV 138.]
Incompatibilities
Contact with nitrates, strong oxidizers;
strong alkalis; strong acids; may pose risk of fire and
explosions. Attacks plastic.
Toxics Screening Level
The final ITSL for n-propyl acetate is 8,350 μg/m3, 8-hour average.
Waste Disposal
Dissolve or mix the
material with a combustible solvent and burn in a chemical
incinerator equipped with an afterburner and scrubber.
All federal, state, and local environmental regulations must
be observed.
References
1.https://en.wikipedia.org/wiki/Propyl_acetate
2.http://www.hmdb.ca/metabolites/HMDB34237
3.http://www.eastman.com/Pages/ProductHome.aspx?product=71001052
4.http://www.khchemicals.com/zh/categories/acetates/n-propyl-acetate/
5.https://www.alfa.com/zh-cn/catalog/L15355/
6.http://product-finder.basf.com/group/corporate/product-finder/en/brand/N_PROPYL_ACETATE