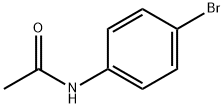
Chemical Properties
4'-Bromoacetanilide is a white to light beige crystalline solid with a melting point of 168°C and a relative density of 1.717. It is soluble in benzene, chloroform, and ethyl acetate, slightly soluble in alcohol and hot water, and insoluble in cold water. This compound can be prepared by brominating acetanilide and is commonly used as an intermediate in organic synthesis.
Uses
4'-Bromoacetanilide is used as an internal standard for phenylurea and the respective metabolic products in oysters. It has also been used as a reagent in the production of a new ligand to form a coordination compex between gadolinium(III) and T=titanium(IV) oxide. It was used in the synthesis of new ligand for anchoring Gd(III) chelates onto TiO2 surface.
Uses
4-Bromoacetanilide was used as internal standard in determination of several phenylurea and triazine herbicides and their transformation products in oyster. It was used in the synthesis of new ligand for anchoring Gd(III) chelates onto TiO
2 surface.
Preparation
Preparation of 4'-Bromoacetanilide from Acetanilide.
Principle: Aromatic compounds can be conveniently brominated by the use of brominating agent, which is normally a solution of bromine in acetic acid. Bromination of activated aromatic compounds usually give 2, 4, 6-tribromo derivatives while moderately activating group like anilide give preferably the para bromo product.
Reaction:
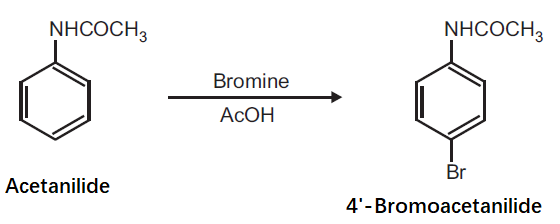
Procedure: Dissolve 0.5 g acetanilide in 0.6 ml glacial acetic acid and add 2.5 ml bromine solution in acetic acid (25% w/v). Shake the mixture for 1 h. After 1 h, pour the mixture on to crushed ice (20 g) with stirring. Filter the separated product and wash with cold water. Dry the product, record the practical yield and re-crystallize it.
Re-crystallization: Dissolve the crude product in minimum amount of ethyl alcohol in a beaker by heating on a water bath. Filter the hot solution and cool the filtrate. The crystals of the product separate out. Filter, dry and record the melting point and TLC (using toluene as a solvent).
Purification Methods
Crystallise the anilide from aqueous MeOH or EtOH. Purify it by zone refining. [Beilstein 12 IV 1504.]