Organic reagents
From early AD to mid-19 century, people mainly use the natural organic substance (such as animal and plant extracts) for qualitative analysis or quantitative analysis. From the second half of the 19th century to the 1920s, it had begun to appear of artificially synthetic organic reagent such as using potassium acetate xanthan for test of nickel, copper, and molybdenum; using morin for test of aluminum; using diazo coupling reaction for the detection of Nitrite; using α-β-nitroso naphthol for detection of cobalt; using dimethyglyoxime for nickel test.
After the proposal of the special-effects group in the 1930s and the proposal of theoretical analysis of functional groups theory in 1950s, people had carried out large-scale screen of organic reagents in search of special-effects analysis groups for different ions and had successfully synthesized a lot of agents of practical value (such as copper reagents, new copper agent, cadmium reagents, beryllium reagent, thorium reagents, etc.). Before the 1950s, the complex compound, in analytic chemistry, is mainly used in the aspects of the precipitation reaction of a binary chelate for the qualitative detection, precipitate isolation and gravimetric separation and other aspects. In the early 1950s and 1960, it is mainly in the form of complexometric titration. From the beginning of the late 1960s, the main focus has been moved to the photometric analysis. Meanwhile, it has been also developed of chelate organic solvent extraction.
8-hydroxy quinoline, hydrazones, oximes, hydroxamic acids, polyphenols, 1, 10-phenanthroline, phosphorus-oxygen extractant, β- diketones, triphenylmethane acid dye, xanthene dyes and azo dyes and other agents has all been widely studied and applied. On the other hand, the structural theoretical research (such as electronic effects, spatial effects and substituent effects) of the organic reagent can further facilitate the development of new-type organic reagents such as heterocyclic azo reagent. During this period, it has been also developed of monoazo chromotropic acid-type and disazo chromotropic acid-type reagent. During the mid-1970s, the chelating resin and adsorbents have achieved rapid development. Since the 1980s, Chinese researchers of analytical chemistry have made progress in the field of the synthesis and application of asymmetric disazo chromotropic acid.
Organic chemical reagents have wide application in analytic chemistry and are mainly used in solvents, precipitation agents, complexing agents, indicator, coloring reagent and surface active agent, etc. In order to adapt to a variety of analytical needs, sometimes it is required to purify certain reagents. For example, liquid organic reagent is often purified by distillation while solid substance is often purified through crystallization and sublimation. Substance having a high vapor pressure, namely having low purification boiling point and undergoing no decomposition under boiling temperature commonly applied atmospheric distillation; for less volatile and water-insoluble substance, namely having high purification point or undergoing decomposition under boiling temperature, we can apply vacuum distillation or steam distillation for purification of it.
Distillation is a commonly used method for laboratory purification of organic reagent. Conventional distillation apparatus generally comprises distiller, condenser and receiver, three parts with auxiliary section also containing heater, a thermometer, a wetted tube and cork.
- Structure:
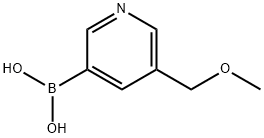
- Chemical Name:5-(METHOXYMETHYL)-3-PYRIDINYL BORONIC ACID
- CAS:200204-95-1
- MF:C7H10BNO3
- Structure:

- Chemical Name:12-bromo-7-phenyltetraphene
- CAS:2883453-97-0
- MF:C24H15Br
- Structure:
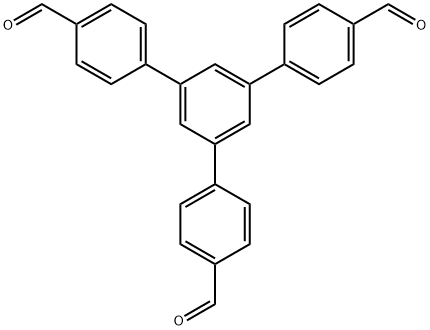
- Chemical Name:1,3,5-Tris(p-formylphenyl)benzene
- CAS:118688-53-2
- MF:C27H18O3
- Structure:
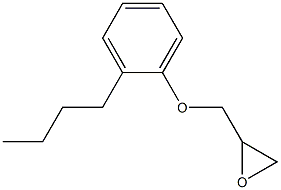
- Chemical Name:P-sec-butyl phenol glycidyl ether
- CAS:29756-52-3
- MF:C13H18O2
- Structure:
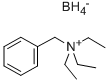
- Chemical Name:BENZYLTRIETHYLAMMONIUM BOROHYDRIDE
- CAS:85874-45-9
- MF:C13H26BN
- Structure:
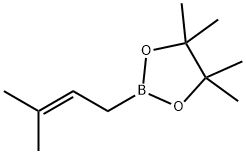
- Chemical Name:3,3-Dimethylallylboronic acid pinacol ester, 2-(3-Methyl-but-2-enyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
- CAS:141550-13-2
- MF:C11H21BO2
- Structure:
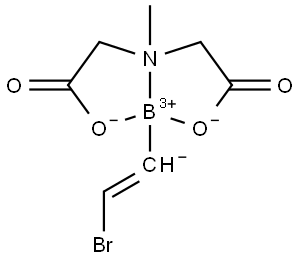
- Chemical Name:BB1
- CAS:1104636-68-1
- MF:C7H9BBrNO4
- Structure:
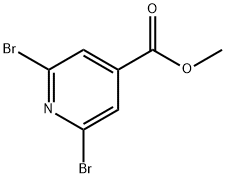
- Chemical Name:Methyl 2,6-dibromopyridine-4-carboxylate
- CAS:119308-57-5
- MF:C7H5Br2NO2
- Chemical Name:NA
- CAS:
- MF:
- Structure:

- Chemical Name:PHENYLACETIC ACID-3-BORONIC ACID PINACOL ESTER
- CAS:
- MF:C14H19BO4
- Structure:
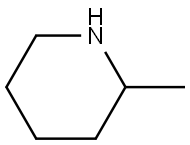
- Chemical Name:2-Methylpiperidine
- CAS:109-05-7
- MF:C6H13N
- Structure:
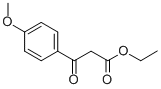
- Chemical Name:ETHYL 4-METHOXYBENZOYLACETATE
- CAS:2881-83-6
- MF:C12H14O4
- Structure:
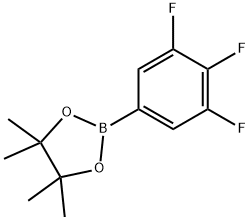
- Chemical Name:3,4,5-TRIFLUOROPHENYLBORONIC ACID, PINACOL ESTER
- CAS:827614-70-0
- MF:C12H14BF3O2
- Structure:
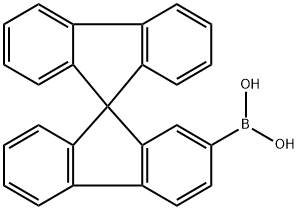
- Chemical Name:9,9'-Spirobi[9H-fluorene]-2-boronic Acid
- CAS:236389-21-2
- MF:C25H17BO2
- Structure:
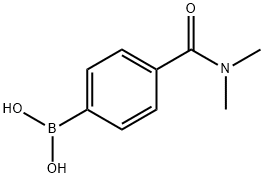
- Chemical Name:4-(N,N-DIMETHYLAMINOCARBONYL)PHENYLBORONIC ACID
- CAS:405520-68-5
- MF:C9H12BNO3
- Structure:
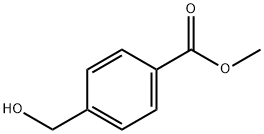
- Chemical Name:METHYL (4-HYDROXYMETHYL)BENZOATE
- CAS:6908-41-4
- MF:C9H10O3
- Structure:
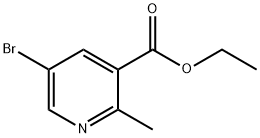
- Chemical Name:5-BROMO-2-METHYL-NICOTINIC ACID ETHYL ESTER
- CAS:129477-21-0
- MF:C9H10BrNO2
- Structure:
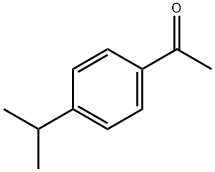
- Chemical Name:4'-Isopropylacetophenone
- CAS:645-13-6
- MF:C11H14O
- Structure:
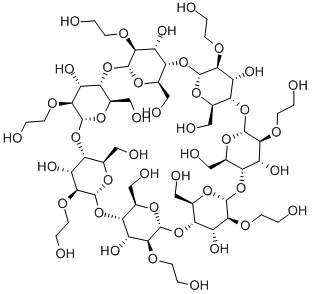
- Chemical Name:HYDROXYETHYL BETA-CYCLODEXTRIN
- CAS:128446-32-2
- MF:C56H98O42
- Structure:
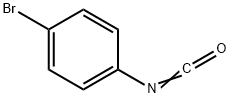
- Chemical Name:4-BROMOPHENYL ISOCYANATE
- CAS:2493-02-9
- MF:C7H4BrNO
- Structure:
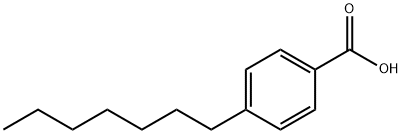
- Chemical Name:4-N-HEPTYLBENZOIC ACID
- CAS:38350-87-7
- MF:C14H20O2
- Structure:
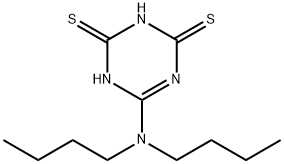
- Chemical Name:6-(DIBUTYLAMINO)-1,3,5-TRIAZINE-2,4-DITHIOL
- CAS:29529-99-5
- MF:C11H20N4S2
- Structure:
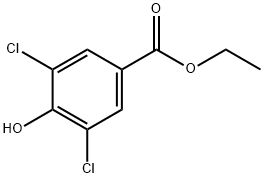
- Chemical Name:ETHYL 3,5-DICHLORO-4-HYDROXYBENZOATE
- CAS:17302-82-8
- MF:C9H8Cl2O3
- Structure:
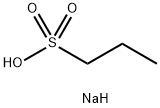
- Chemical Name:Sodium 1-propanesulfonate
- CAS:14533-63-2
- MF:C3H9NaO3S
- Structure:
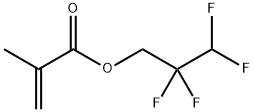
- Chemical Name:2,2,3,3-Tetrafluoropropyl methacrylate
- CAS:45102-52-1
- MF:C7H8F4O2
- Structure:
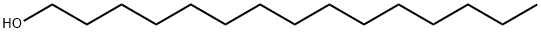
- Chemical Name:1-Pentadecanol
- CAS:629-76-5
- MF:C15H32O
- Structure:
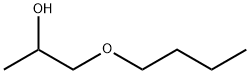
- Chemical Name:1-BUTOXY-2-PROPANOL
- CAS:5131-66-8
- MF:C7H16O2
- Structure:
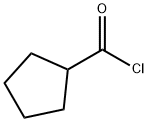
- Chemical Name:Cyclopentanecarbonyl chloride
- CAS:4524-93-0
- MF:C6H9ClO
- Structure:
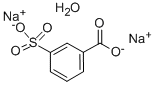
- Chemical Name:3-SULFOBENZOIC ACID DISODIUM SALT MONOHYDRATE
- CAS:14995-40-5
- MF:C7H6Na2O6S
- Structure:
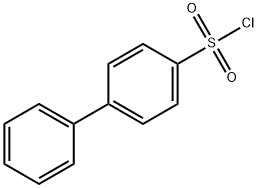
- Chemical Name:4-BIPHENYLSULFONYL CHLORIDE
- CAS:1623-93-4
- MF:C12H9ClO2S
- Structure:
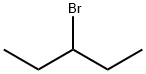
- Chemical Name:3-Bromopentane
- CAS:1809-10-5
- MF:C5H11Br
- Structure:
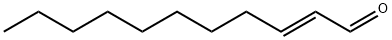
- Chemical Name:TRANS-2-UNDECENAL
- CAS:53448-07-0
- MF:C11H20O
- Structure:
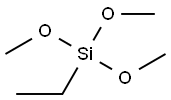
- Chemical Name:ETHYLTRIMETHOXYSILANE
- CAS:5314-55-6
- MF:C5H14O3Si
- Structure:

- Chemical Name:4,7,10-TRIOXA-1,13-TRIDECANEDIAMINE
- CAS:4246-51-9
- MF:C10H24N2O3
- Structure:
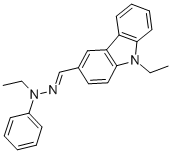
- Chemical Name:9-ETHYL-3-(N-ETHYL-N-PHENYLHYDRAZONOMETHYL)CARBAZOLE
- CAS:84678-52-4
- MF:C23H23N3
- Structure:
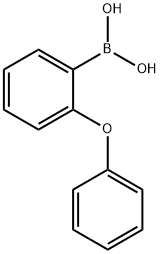
- Chemical Name:2-PHENOXYPHENYLBORONIC ACID
- CAS:108238-09-1
- MF:C12H11BO3
- Structure:
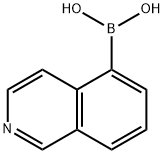
- Chemical Name:Isoquinoline-5-boronic acid
- CAS:371766-08-4
- MF:C9H8BNO2
- Structure:

- Chemical Name:AZELANITRILE
- CAS:1675-69-0
- MF:C9H14N2
- Structure:
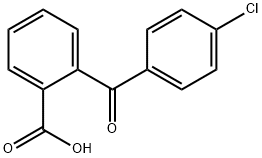
- Chemical Name:2-(4-Chlorobenzoyl)benzoic acid
- CAS:85-56-3
- MF:C14H9ClO3
- Structure:
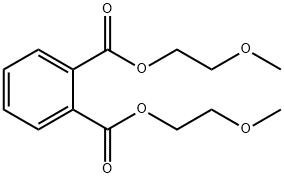
- Chemical Name:Bis(2-methoxyethyl) phthalate
- CAS:117-82-8
- MF:C14H18O6
- Structure:
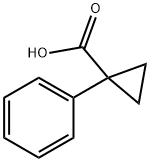
- Chemical Name:1-Phenyl-1-cyclopropanecarboxylic acid
- CAS:6120-95-2
- MF:C10H10O2
- Structure:
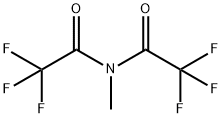
- Chemical Name:N-Methyl-bis(trifluoroacetamide)
- CAS:685-27-8
- MF:C5H3F6NO2
- Structure:
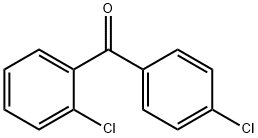
- Chemical Name:2,4'-Dichlorobenzophenone
- CAS:85-29-0
- MF:C13H8Cl2O
- Structure:
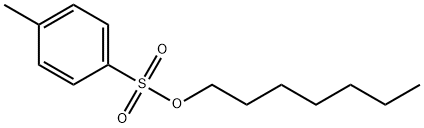
- Chemical Name:Heptyl p-Toluenesulfonate
- CAS:24767-82-6
- MF:C14H22O3S
- Structure:
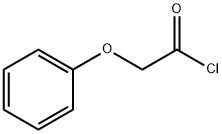
- Chemical Name:Phenoxyacetyl chloride
- CAS:701-99-5
- MF:C8H7ClO2
- Structure:
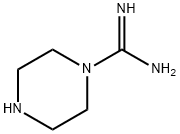
- Chemical Name:PIPERAZINE-1-CARBOXAMIDINE
- CAS:45695-84-9
- MF:C5H12N4
- Structure:
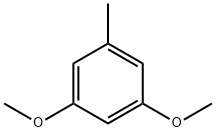
- Chemical Name:3,5-Dimethoxytoluene
- CAS:4179-19-5
- MF:C9H12O2
- Structure:
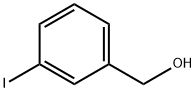
- Chemical Name:3-IODOBENZYL ALCOHOL
- CAS:57455-06-8
- MF:C7H7IO
- Structure:
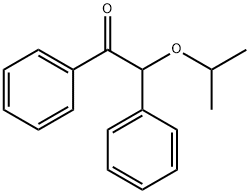
- Chemical Name:BENZOIN ISOPROPYL ETHER
- CAS:6652-28-4
- MF:C17H18O2
- Structure:
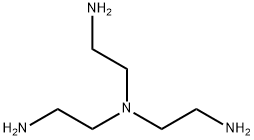
- Chemical Name:Tris(2-aminoethyl)amine
- CAS:4097-89-6
- MF:C6H18N4
- Structure:
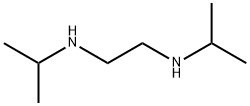
- Chemical Name:N,N'-Diisopropylethylenediamine
- CAS:4013-94-9
- MF:C8H20N2
- Structure:
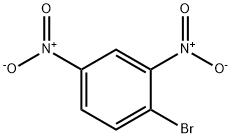
- Chemical Name:1-BROMO-2,4-DINITROBENZENE
- CAS:584-48-5
- MF:C6H3BrN2O4
- Structure:
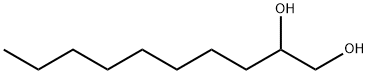
- Chemical Name:1,2-Decanediol
- CAS:1119-86-4
- MF:C10H22O2
- Structure:
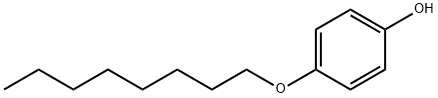
- Chemical Name:4-Octyloxyphenol
- CAS:3780-50-5
- MF:C14H22O2
- Structure:
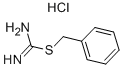
- Chemical Name:2-Benzyl-2-thiopseudourea hydrochloride
- CAS:538-28-3
- MF:C8H11ClN2S
- Structure:
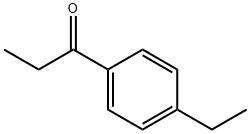
- Chemical Name:4'-Ethylpropiophenone
- CAS:27465-51-6
- MF:C11H14O
- Structure:
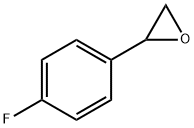
- Chemical Name:2-(4-FLUOROPHENYL)OXIRANE
- CAS:18511-62-1
- MF:C8H7FO
- Structure:
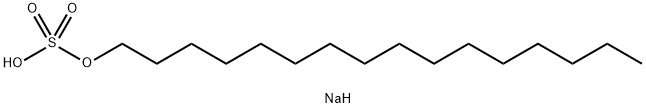
- Chemical Name:N-HEXADECYLSULFURIC ACID SODIUM SALT
- CAS:1120-01-0
- MF:C16H35NaO4S
- Structure:
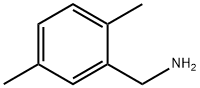
- Chemical Name:2,5-Dimethylbenzylamine
- CAS:93-48-1
- MF:C9H13N
- Structure:
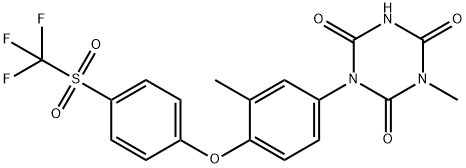
- Chemical Name:Ponazuril
- CAS:69004-04-2
- MF:C18H14F3N3O6S
- Structure:
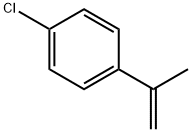
- Chemical Name:4-Chloro-alpha-methylstyrene
- CAS:1712-70-5
- MF:C9H9Cl
- Structure:
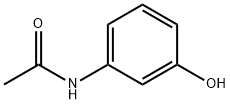
- Chemical Name:3-ACETAMIDOPHENOL
- CAS:621-42-1
- MF:C8H9NO2
- Structure:
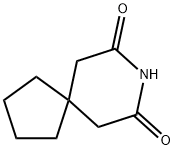
- Chemical Name:3,3-Tetramethyleneglutarimide
- CAS:1075-89-4
- MF:C9H13NO2
- Structure:
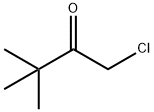
- Chemical Name:1-Chloropinacolone
- CAS:13547-70-1
- MF:C6H11ClO
- Structure:
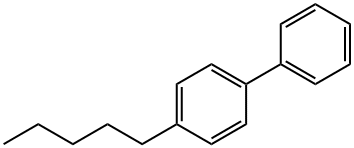
- Chemical Name:4-N-PENTYLBIPHENYL
- CAS:7116-96-3
- MF:C17H20
- Structure:
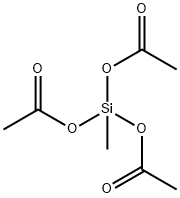
- Chemical Name:Methyltriacetoxysilane
- CAS:4253-34-3
- MF:C7H12O6Si
- Structure:
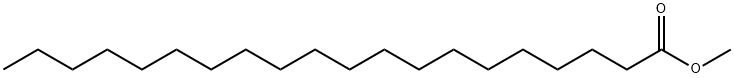
- Chemical Name:Methyl arachidate
- CAS:1120-28-1
- MF:C21H42O2
- Structure:
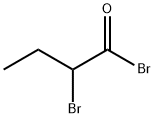
- Chemical Name:2-Bromobutyryl bromide
- CAS:26074-52-2
- MF:C4H6Br2O
- Structure:
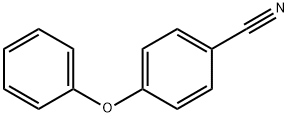
- Chemical Name:4-PHENOXYBENZONITRILE
- CAS:3096-81-9
- MF:C13H9NO
- Structure:
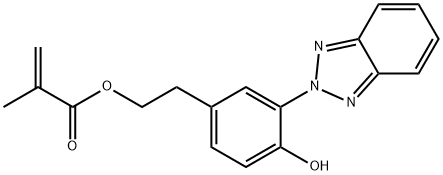
- Chemical Name:2-[3-(2H-Benzotriazol-2-yl)-4-hydroxyphenyl]ethyl methacrylate
- CAS:96478-09-0
- MF:C18H17N3O3
- Structure:
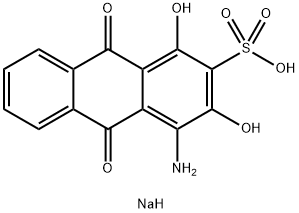
- Chemical Name:NUCLEAR FAST RED
- CAS:6409-77-4
- MF:C14H10NNaO7S
- Structure:
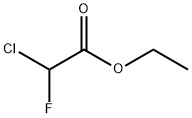
- Chemical Name:ETHYL CHLOROFLUOROACETATE
- CAS:401-56-9
- MF:C4H6ClFO2
- Structure:
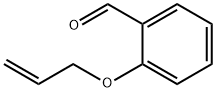
- Chemical Name:2-ALLYLOXYBENZALDEHYDE
- CAS:28752-82-1
- MF:C10H10O2
- Structure:
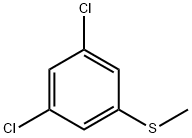
- Chemical Name:3,5-DICHLOROTHIOANISOLE
- CAS:68121-46-0
- MF:C7H6Cl2S
- Structure:
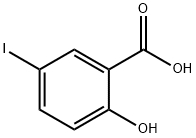
- Chemical Name:5-Iodosalicylic acid
- CAS:119-30-2
- MF:C7H5IO3
- Structure:
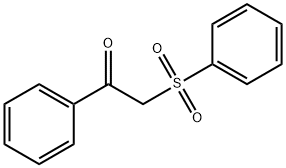
- Chemical Name:2-(PHENYLSULFONYL)ACETOPHENONE
- CAS:3406-03-9
- MF:C14H12O3S
- Structure:
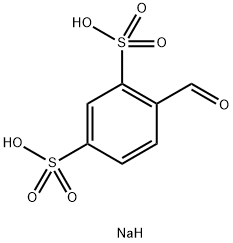
- Chemical Name:Benzaldehyde-2,4-disulfonic acid disodium salt
- CAS:33513-44-9
- MF:C7H7NaO7S2
- Structure:
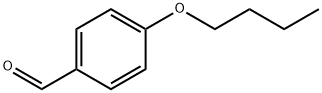
- Chemical Name:4-N-BUTOXYBENZALDEHYDE
- CAS:5736-88-9
- MF:C11H14O2
- Structure:
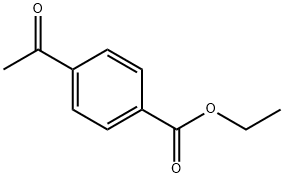
- Chemical Name:ETHYL 4-ACETYLBENZOATE
- CAS:38430-55-6
- MF:C11H12O3
- Structure:
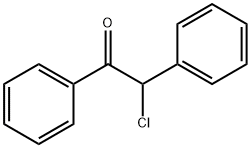
- Chemical Name:DESYL CHLORIDE
- CAS:447-31-4
- MF:C14H11ClO
- Structure:
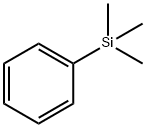
- Chemical Name:PHENYLTRIMETHYLSILANE
- CAS:768-32-1
- MF:C9H14Si
- Structure:
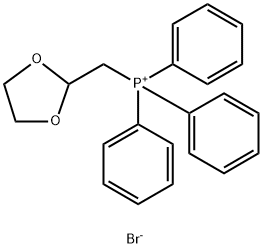
- Chemical Name:(1,3-Dioxolan-2-ylmethyl)triphenylphosphonium bromide
- CAS:52509-14-5
- MF:C22H22BrO2P
- Structure:
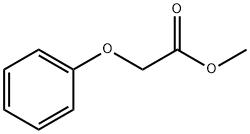
- Chemical Name:Methyl phenoxyacetate
- CAS:2065-23-8
- MF:C9H10O3
- Structure:
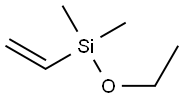
- Chemical Name:Ethoxydimethylvinylsilane
- CAS:5356-83-2
- MF:C6H14OSi
- Structure:
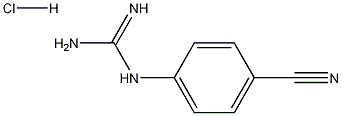
- Chemical Name:N-(4-Cyanophenyl)guanidine hydrochloride
- CAS:373690-68-7
- MF:C8H8N4.HCl
- Structure:
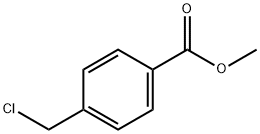
- Chemical Name:Methyl 4-(chloromethyl)benzoate
- CAS:34040-64-7
- MF:C9H9ClO2
- Structure:
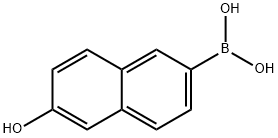
- Chemical Name:6-HYDROXY-2-NAPHTHALENEBORONIC ACID
- CAS:173194-95-1
- MF:C10H9BO3
- Structure:
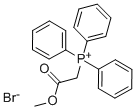
- Chemical Name:(Carbomethoxymethyl)triphenylphosphonium bromide
- CAS:1779-58-4
- MF:C21H20BrO2P
- Structure:
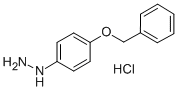
- Chemical Name:4-Benzyloxyphenylhydrazine hydrochloride
- CAS:52068-30-1
- MF:C13H15ClN2O
- Structure:
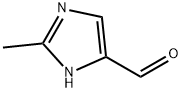
- Chemical Name:2-Methyl-1H-imidazole-4-carbaldehyde
- CAS:35034-22-1
- MF:C5H6N2O
- Structure:
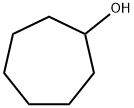
- Chemical Name:Cycloheptanol
- CAS:502-41-0
- MF:C7H14O
- Structure:
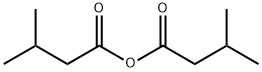
- Chemical Name:ISOVALERIC ANHYDRIDE
- CAS:1468-39-9
- MF:C10H18O3
- Structure:

- Chemical Name:3-Chloropropyl isocyanate
- CAS:13010-19-0
- MF:C4H6ClNO
- Structure:
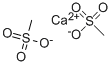
- Chemical Name:METHANESULFONIC ACID CALCIUM SALT
- CAS:58131-47-8
- MF:C2H6CaO6S2
- Structure:
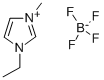
- Chemical Name:1-Ethyl-3-methylimidazolium tetrafluoroborate
- CAS:143314-16-3
- MF:C6H11BF4N2
- Structure:
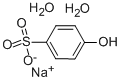
- Chemical Name:4-HYDROXYBENZENESULFONIC ACID SODIUM SALT DIHYDRATE
- CAS:10580-19-5
- MF:C6H9NaO6S
- Structure:
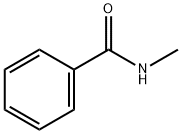
- Chemical Name:N-Methylbenzamide
- CAS:613-93-4
- MF:C8H9NO
- Structure:
- Chemical Name:2-IODO-2-METHYLPROPANE
- CAS:558-17-8
- MF:C4H9I
- Structure:
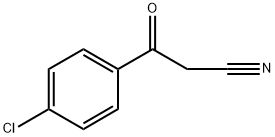
- Chemical Name:4-CHLOROBENZOYLACETONITRILE
- CAS:4640-66-8
- MF:C9H6ClNO
- Structure:
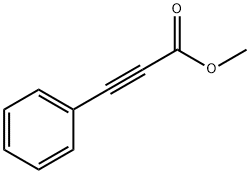
- Chemical Name:METHYL PHENYLPROPIOLATE
- CAS:4891-38-7
- MF:C10H8O2