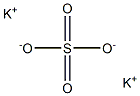
Калий сернокислый
- английское имяPotassium sulfate
- CAS №7778-80-5
- CBNumberCB9854321
- ФормулаK2O4S
- мольный вес174.2592
- EINECS231-915-5
- номер MDLMFCD00011388
- файл Mol7778-80-5.mol
| Температура плавления | 1067°C | ||||||||||||||
| Температура кипения | 1689°C | ||||||||||||||
| Плотность накопления | 800kg/m3 | ||||||||||||||
| плотность | 2.66 | ||||||||||||||
| Fp | 1689°C | ||||||||||||||
| температура хранения | Inert atmosphere,Room Temperature | ||||||||||||||
| растворимость | H2O: 0.5 M at 20 °C, clear, colorless | ||||||||||||||
| форма | Very Fine Crystals or Powder | ||||||||||||||
| цвет | White | ||||||||||||||
| Удельный вес | 2.662 | ||||||||||||||
| Запах | Odorless | ||||||||||||||
| Водородный показатель | ~7 | ||||||||||||||
| РН | 5.5-7.5 (25℃, 0.5M in H2O) | ||||||||||||||
| Биологические источники | synthetic | ||||||||||||||
| Растворимость в воде | 110 g/L (20 ºC) | ||||||||||||||
| λмакс | λ: 260 nm Amax: 0.01 λ: 280 nm Amax: 0.01 |
||||||||||||||
| Мерк | 14,7674 | ||||||||||||||
| crystal system | Nogata | ||||||||||||||
| Space group | Pnma | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Диэлектрическая постоянная | 5.9(0.0℃) | ||||||||||||||
| Стабильность | Stable. | ||||||||||||||
| ИнЧИКей | OTYBMLCTZGSZBG-UHFFFAOYSA-L | ||||||||||||||
| Справочник по базе данных CAS | 7778-80-5(CAS DataBase Reference) | ||||||||||||||
| FDA 21 CFR | 184.1643; 582.1643; 558.311 | ||||||||||||||
| Вещества, добавляемые в пищу (ранее EAFUS) | POTASSIUM SULFATE | ||||||||||||||
| Рейтинг продуктов питания EWG | 1 | ||||||||||||||
| Словарь онкологических терминов NCI | SOP | ||||||||||||||
| FDA UNII | 1K573LC5TV | ||||||||||||||
| Справочник по химии NIST | Potassium sulfate(7778-80-5) | ||||||||||||||
| Система регистрации веществ EPA | Potassium sulfate (7778-80-5) | ||||||||||||||
| UNSPSC Code | 12352302 | ||||||||||||||
| NACRES | NB.24 |
| Заявления о безопасности | 22-24/25 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | TT5900000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 31043000 | |||||||||
| Банк данных об опасных веществах | 7778-80-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 6600 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P262:Избегать попадания в глаза, на кожу или одежду.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Калий сернокислый химические свойства, назначение, производство
Описание
Potassium sulfate (K2SO4) is a kind of chemical compounds that is commonly used in agriculture. The dominant application of potassium sulfate is as a fertilizer, which is commonly applied to offer both potassium and sulfur, thus improving the quality and yield of crops growing in soils that lack an adequate supply of this essential elements. Besides, the crude potassium sulfate is sometimes employed in the production of glass. It also has applications in other industries, which is used as a flash reducer in artillery propellant charges and as an alternative blast media similar to soda in the process of soda blasting.
potassium sulfate powder
Химические свойства
Potassium sulfate,K2804, also known as salt of Lemery and arcanite, is a colorless crystalline solid that melts at 1072°C(1960 OF). It is soluble in water,but insoluble in alcohol. Potassium sulfate is used in manufacturing glass, aluminum, fertilizers, and in medicine.Физические свойства
Colorless or white crystals or white granules or powder; rhombohedral structure; bitter taste; density 2.66 g/cm3; melts at 1,069°C; vaporizes at 1,689°C; moderately soluble in water, 12 g/100mL at 25°C and 24g/100mL at 100°C; slightly soluble in glycerol; insoluble in alcohol, acetone, and carbon disulfide.Вхождение
Potassium and sodium sulfates and their double sulfates with calcium and magnesium occur naturally in various salt lakes. Potassium sulfate also occurs in certain volcanic lava. Its double salt with magnesium occurs in nature, as the mineral langbeinite.Potassium sulfate is used in fertilizers as a source of potassium and sulfur, both of which are essential elements for plant growth. Either in sim-ple form or as a double salt with magnesium sulfate, potassium sulfate is one of the most widely consumed potassium salts in agricultural applications. It is preferred over potassium chloride for certain types of crops; such as, tobac-co, citrus, and other chloride-sensitive crops. Some other applications include making gypsum cements; to make potassium alum; in the analysis of Kjeldahl nitrogen; and in medicine.
Использование
potassium sulfate is a reagent in cosmetics. Potassium sulfate is an inorganic salt with a primary function as a viscosity-increasing agent.Методы производства
Potassium sulfate is produced by various methods, selection of process depending on availability and cost of raw materials.The salt may be obtained from its naturally occurring mineral, langbeinite,K2SO4?2MgSO4. The ore first is crushed and washed with water to separate sodium chloride. After that, magnetite is separated from the washed langbei-nite by magnetic separation. After the separation of these two major impuri-ties, the purified double salt is treated with an aqueous solution of potassium chloride to obtain potassium sulfate:
K2SO4?2MgSO4 + 4KCl →3K2SO4+ 2MgCl2
The solution is filtered to remove insoluble residues and the products are separated from their aqueous mixture by crystallization.
Potassium sulfate also is produced from the mineral kieserite, MgSO4?H2O by treatment with potassium chloride. The intermediate double salt obtained reacts further with potassium chloride to form potassium sulfate:MgSO4?H2O + 2KCl + 4H2O →K2SO4?MgSO4?6H2O + MgCl2
K2SO4?MgSO4?6H2O + 2KCl →2K2SO4+ MgCl2
Potassium sulfate is separated from the more soluble magnesium chloride by crystallization.
Also, potassium sulfate can be made by two other processes in which no naturally occurring mineral is employed. In the Mannheim process, the salt is produced by action of sulfuric acid on potassium chloride:2KCl + H2SO4→K2SO4+ 2HCl
In Hargreaves process, which is a slight variation of the Mannheim method, potassium sulfate is made by heating a mixture of potassium chlo-ride, sulfur dioxide, air and water:4KCl + 2SO2+ 2H2O + o2→2K2SO4+ 4HCl.
Определение
Potassium sulfate is a potassium salt and an inorganic potassium salt. It is a white crystalline powder, K2SO4, soluble inwater and insoluble in ethanol;rhombic or hexagonal; r.d. 2.66; m.p.1069°C. It occurs naturally assch nite (Strassfurt deposits) and inlake brines, from which it is separatedby fractional crystallization. Ithas also been produced by the Hargreavesprocess, which involves theoxidation of potassium chloride withsulphuric acid. In the laboratory itmay be obtained by the reaction ofeither potassium hydroxide or potassiumcarbonate with sulphuric acid.Potassium sulphate is used in cements,in glass manufacture, as a food additive, and as a fertilizer(source of K+) for chloride-sensitiveplants, such as tobacco and citrus.Общее описание
Potassium sulfate is an inorganic salt that can be prepared by reacting phosphogypsum and potassium chloride. It forms needlelike mullite particles on heating with aluminum sulfate (Al2(SO4)3) and silicon dioxide (SiO2). Its application to the soil has been reported to minimize the bronzing of rice plants. Its surface integration growth kinetics has been obtained in the temperature range of 20-50°C. The theoretical heat capacity curve of potassium sulfate in vapor phase has been obtained.Профиль безопасности
Moderately toxic to humans by ingestion. Moderately toxic experimentally by subcutaneous route. Swallowing large doses causes severe gastrointestinal tract effects. When heated to decomposition it emits toxic fumes of K2O and SOx. See also SULFATES.Методы очистки
Potassium sulfate [7778-80-5] M 174.3, m 1069o, d 4 2.67 It crystallised from distilled water (4mL/g at 20o; 8mL/g at 100o) between 100o and 0o.использованная литература
https://en.wikipedia.org/wiki/Potassium_sulfatehttp://www.cropnutrition.com/potassium-sulfate
http://study.com/academy/lesson/what-is-potassium-sulfate-structure-uses-formula.html
Калий сернокислый запасные части и сырье
сырьё
1of2
запасной предмет
- Compound fertilizer
- Алюминия калия сульфат додекагидра
- ethyl 3-ethylpyrazolyl-5-carboxylate
- 4-гидроксибензойной кислоты
- Аценафталин
- АКРОЛЕИН
- 4-[2-(diethylamino)ethoxy]benzophenone
- Nitrogen phosphorus potassium mixed fertilizer
- Калий сернокислый пиро
- Nitrogen-Phosphorus-Potassium compound fertilizer with sulphur
- Calcium phosphate monobasic
- Nicosulfuron
- N-(1,3-ДИМЕТИЛБУТИЛ)-N'-ФЕНИЛ-p-ФЕНИЛЕНДИАМИН
- Калия гидрокарбонат
- Mixed and compound fertilizer
- Калий метабисульфит
- ПировиноGRадная кислота
- Бромат калия
- Кальция гидрофосфат
- АЛЬФА-D-ГЛЮКОПИРАНОЗА 1-ФОСФАТ ДИПОТАССИЕВОЙ СОЛИ ГИДРАТ
1of6
Калий сернокислый поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +86-15271838296; +8615271838296 |
China | 1512 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | |
| +86-86-17798073498 +8617798073498 |
China | 299 | 58 | |
| +86-18532138899 +86-18532138899 |
China | 939 | 58 |