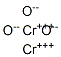
Хром (III) оксид
- английское имяChromium(III) oxide
- CAS №1308-38-9
- CBNumberCB8394705
- ФормулаCr2O3
- мольный вес151.99
- EINECS215-160-9
- номер MDLMFCD00010949
- файл Mol1308-38-9.mol
химическое свойство
| Температура плавления | 2435 °C | ||||||||||||||
| Температура кипения | 4000 °C | ||||||||||||||
| плотность | 5.21 | ||||||||||||||
| показатель преломления | 2.551 | ||||||||||||||
| Fp | 3000°C | ||||||||||||||
| температура хранения | Room Temperature | ||||||||||||||
| растворимость | Insoluble in all solvents | ||||||||||||||
| форма | powder | ||||||||||||||
| цвет | Pale to dark green | ||||||||||||||
| Удельный вес | 5.22 | ||||||||||||||
| Запах | at 100.00?%. odorless | ||||||||||||||
| Растворимость в воде | Insoluble | ||||||||||||||
| Кристальная структура | Trigonal | ||||||||||||||
| crystal system | Three sides | ||||||||||||||
| Мерк | 14,2234 | ||||||||||||||
| Space group | R3c | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Пределы воздействия | NIOSH: IDLH 25 mg/m3; TWA 0.5 mg/m3 | ||||||||||||||
| Стабильность | Stable. | ||||||||||||||
| Справочник по базе данных CAS | 1308-38-9(CAS DataBase Reference) | ||||||||||||||
| FDA 21 CFR | 73.1327; 73.2327; 73.3111 | ||||||||||||||
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | CHROMIUM OXIDE GREEN | ||||||||||||||
| Рейтинг продуктов питания EWG | 1 | ||||||||||||||
| FDA UNII | X5Z09SU859 | ||||||||||||||
| Справочник по химии NIST | Chromium(iii) oxide(1308-38-9) | ||||||||||||||
| Система регистрации веществ EPA | Chromium(III) oxide (1308-38-9) | ||||||||||||||
| UNSPSC Code | 12352303 | ||||||||||||||
| NACRES | NA.55 |
больше
| Заявления о безопасности | 24/25 | |||||||||
| WGK Германия | - | |||||||||
| RTECS | GB6475000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28199090 | |||||||||
| Банк данных об опасных веществах | 1308-38-9(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H332:Вредно при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
Хром (III) оксид химические свойства, назначение, производство
Описание
Chromium(III) oxide is among the ten most abundant compounds in the Earth's crust. It is one of four oxides of chromium, chemical formula Cr2O3. It is commonly called "chrome green" when used as a pigment; however it was referred to as “viridian” when it was first discovered.
Chromium(III) oxide is a very refractory ceramic colorant (even a 50% mix with a high borax frit will not even begin to melt it in a crucible). Chrome oxide is the only stable oxide of the metal chromium. It is a bright to dark green crystalline powder insoluble in alkalis and acids. It is manufactured from the mineral Chromite mined in southern Africa, Asia, Turkey and Cuba. As with other powerful coloring agents, chrome must be milled fine enough to eliminate specking in glass or glaze.
Chromium is a "fast" colorant, meaning can produce strong green colors under all furnace conditions, slow or fast, reducing or oxidizing. It is also a flat colorant (due to its refractory nature), it usually produces an army helmet opaque green. It is powerful, typically only 2% will produce a dark color. It cannot be used to make a metallic glaze.
Chrome oxide is usually employed in raw glazes whereas potassium dichromate is used in fritted glazes.
Химические свойства
Chromium oxide is a bright green, odorless powder. Chromium(III) oxide pigments are thermally stable and insoluble in water.Chromium oxide pigments, also called chromium oxide green pigments, consist of chromium(III) oxide [1308-38-9], Cr2O3,Mr 151.99. Chromium oxide green is one of the few single-component pigments with green coloration. Chrome green is a blend of chrome yellow and iron blue pigments; phthalochrome green is a blend of chrome yellow and blue phthalocyanine pigments.
Alkali dichromates are used as starting materials for the production of chromium(III) oxide pigments. They are not classified as hazardous materials and are not subject to international transport regulations. As long as they are kept dry their utility as a pigment is practically unlimited.
Физические свойства
Green hexagonal crystal system; corundum type structure; density 5.22 g/cm3; melts at 2,330°C; vaporizes above 3,000°C; insoluble in water and alcohol.Использование
Chromium(III) oxide is used as pigment for coloring green on glass and fabrics. Other important applications are in metallurgy; as a component of refractory bricks, abrasives and ceramics; and as a catalyst in hydrogenation, hydrogenolysis and many other organic conversion reactions. It also is used to prepare other chromium salts.Подготовка
Chromium(III) oxide can be obtained by thermal decomposition of ammonium dichromate. Above ca. 200 °C, a highly voluminous product is formed with elimination of nitrogen. The pigment is obtained after addition of alkali salts (e.g., sodium sulfate) and subsequent calcination.In the industrial process, a mixture of ammonium sulfate or chloride and sodium dichromate is calcined:
Na2Cr2O7.2 H2O + (NH4)2SO4 →Cr2O3 + Na2SO4 + 6 H2O + N2
Определение
chromium sesquioxide: A green crystallinewater-insoluble salt, Cr2O3; r.d. 5.21;m.p. 2435°C; b.p. 4000°C. It is obtainedby heating chromium in astream of oxygen or by heating ammoniumdichromate. The industrialpreparation is by reduction ofsodium dichromate with carbon.Chromium(III) oxide is amphoteric,dissolving in acids to give chromium(III) ions and in concentratedsolutions of alkalis to give chromites.It is used as a green pigment in glass,porcelain, and oil paint.Реакции
Chromium(III) oxide is amphoteric. Although insoluble in water, it dissolves in acid to produce hydrated chromium ion, [Cr(H2O)6]3+. It dissolves in concentrated alkali to yield chromite ion. When heated with finely divided aluminum or carbon it is reduced to chromium metal:Cr2O3 + 3Al2Cr + Al2O3
Heating with chlorine and carbon yields chromium(III) chloride:
Cr2O3 + 3Cl2 + 3C2CrCl3 + 3CO
If chromium(III) oxide (also known as chrome green) is heated with potassium carbonate and potassium nitrate, the mixture slowly turns yellow. This colour change stems from the formation of potassium chromate, K2CrO4, in which chromium is found in oxidation state vi.
Общее описание
Chromium (III) oxide is a chromium complex in which the chromium ion is in +3 oxidation state. The synthesis of its sub-micron powder has been reported.? The IR and Raman spectra of chromium (III) oxide have been studied.? The impact of adding lysozyme on the stability of chromium (III) oxide (Cr2O3) suspension has been evaluated.?Опасность
Toxic by ingestion and inhalation.Профиль безопасности
Confirmed carcinogen with experimental tumorigenic data. Mutation data reported. Probably a severeВозможный контакт
Chromium(III) oxide is used as a paint pigment, a fixative for certain textile dyes; in the manufacture of chromium; and a catalyst.Перевозки
UN3086 Toxic solids, oxidizing, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, 5.1-Oxidizer. Technical Name Required. Spill Handling: Evacuate persons not wearing protective equipment from the danger area of spill or leak until cleanup is complete. Remove all ignition sources. Collect powdered material in the most convenient and safe manner and deposit in sealed containers. Ventilate area after clean-up is complete. It may be necessary to contain and dispose of this chemical as a hazardous waste. If material or contaminated runoff enters waterways, notify downstream users of potentially contaminated waters. Contact your local or federal environmental protection agency for specific recommendations. If employees are required to clean-up spills, they must be properly trained and equipped. OSHA 1910.120(q) may be applicable.Структура и конформация
Chromium oxide is a dense, crystalline material, density 5.2 g/cm3, of the corundum type. It is quite hard-abut 9 on the Moh scale-which makes it a good grinding material, but too abrasive for many pigment applications. The particle size depends on the manufacturing process, but it is distributed around mean values in the range 0.5-0.6 μm. The refractive index of chromium oxide is quite high, 2.5, which is almost as high as that of rutile TiO2, 2.7. Chromium oxide pigments are green with an olive green tint. Small particles are lighter green with a yellowish hue, whereas larger particles are darker, with a bluish tint. Chromium oxide pigments are very inert materials with outstanding lightfastness and excellent resistance to acids, alkalis, and high temperatures.Несовместимости
A strong oxidizer. Contact with reducing agents; organics, and combustibles may be violentХром (III) оксид запасные части и сырье
сырьё
- ChroMiuM hydroxide
- Натрий дихромат дигидрат
- Железо (III) гексацианоферрат (II)
- Sulfur dioxide
- Хром(VI) оксид
- Sulfur trioxide
- КАЛИЙ двухромовокислый
- Middle chrome yellow
- Lead chrome yellow pigment
- Меди (II) фталоцианин
1of5
запасной предмет
Хром (III) оксид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +8617798174412 | China | 2097 | 58 | |
| +8619931165850 | China | 1000 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 |
Хром (III) оксид Обзор)
1of4