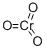
Хром(VI) оксид
- английское имяChromium(VI) oxide
- CAS №1333-82-0
- CBNumberCB5304358
- ФормулаCrO3
- мольный вес99.99
- EINECS215-607-8
- номер MDLMFCD00010952
- файл Mol1333-82-0.mol
| Температура плавления | 196 °C (dec.)(lit.) | ||||||||||||||
| Температура кипения | 330 °C | ||||||||||||||
| плотность | 2.7 | ||||||||||||||
| Плотность накопления | 900kg/m3 | ||||||||||||||
| давление пара | 0Pa at 25℃ | ||||||||||||||
| Fp | 250°C | ||||||||||||||
| температура хранения | Store below +30°C. | ||||||||||||||
| растворимость | 1.667g/l | ||||||||||||||
| форма | macroporous | ||||||||||||||
| пка | -0.61[at 20 ℃] | ||||||||||||||
| Удельный вес | 2.7 | ||||||||||||||
| цвет | Reddish-violet | ||||||||||||||
| РН | <1 (100g/l, H2O, 20℃) | ||||||||||||||
| Растворимость в воде | Highly soluble | ||||||||||||||
| Точка замерзания | 170~172℃ | ||||||||||||||
| Чувствительный | Hygroscopic | ||||||||||||||
| Мерк | 14,2235 | ||||||||||||||
| crystal system | Nogata | ||||||||||||||
| Space group | Ama2 | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Пределы воздействия | ACGIH: TWA 0.0002 mg/m3; STEL 0.0005 mg/m3 (Skin) OSHA: Ceiling 0.1 mg/m3 NIOSH: IDLH 15 mg/m3; TWA 0.0002 mg/m3 |
||||||||||||||
| Стабильность | Stable. Strong oxidizer. Reacts with most organic material in a violent and often explosive fashion. Moisture sensitive. | ||||||||||||||
| ИнЧИКей | WGLPBDUCMAPZCE-UHFFFAOYSA-N | ||||||||||||||
| Справочник по базе данных CAS | 1333-82-0(CAS DataBase Reference) | ||||||||||||||
| Рейтинг продуктов питания EWG | 8-10 | ||||||||||||||
| FDA UNII | 8LV49809UC | ||||||||||||||
| Справочник по химии NIST | Chromium trioxide(1333-82-0) | ||||||||||||||
| Система регистрации веществ EPA | Chromium(VI) trioxide (1333-82-0) | ||||||||||||||
| UNSPSC Code | 12352303 | ||||||||||||||
| NACRES | NB.24 |
| Коды опасности | O,T+,N,T | |||||||||
| Заявления о рисках | 45-46-9-24/25-26-35-42/43-48/23-50/53-62-8 | |||||||||
| Заявления о безопасности | 53-45-60-61-36/37/39-28-26-22 | |||||||||
| РИДАДР | UN 1463 5.1/PG 2 | |||||||||
| OEB | E | |||||||||
| OEL | TWA: 0.0002 mg/m3 (8-hours) | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | GB6650000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 5.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28191000 | |||||||||
| Банк данных об опасных веществах | 1333-82-0(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 oral (rat) 80 mg/kg PEL (OSHA) 0.1 mg (CrO3)/m3 (ceiling) TLV-TWA (ACGIH) 0.05 mg (Cr)/m3 | |||||||||
| ИДЛА | 15 mg Cr(VI)/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)





-
сигнальный язык
опасность
-
вредная бумага
H335:Может вызывать раздражение верхних дыхательных путей.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H350:Может вызывать раковые заболевания.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H330:Смертельно при вдыхании.
H334:При вдыхании может вызывать аллергическую реакцию (астму или затрудненное дыхание).
H361f:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению.
H301+H311:Токсично при проглатывании или при контакте с кожей.
H340:Может вызывать генетические дефекты.
H271:Сильный окислитель; может вызвать возгорание или взрыв.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Хром(VI) оксид химические свойства, назначение, производство
Описание
Chromium hydroxide (Cr2O(OH)4) is a bright bluish-green pigment prepared by the calcinations of bichromate with boric acid at 500°C. The mass during cooling is hydrolyzed with water, which yields the hydrate.Химические свойства
Chromium trioxide is a dark-red crystalline substance. It is odorlessФизические свойства
Dark-red crystals, flakes or granular powder; bipyramidal prismatic system; density 2.70 g/cm3; melts at 197°C; decomposes on further heating; highly soluble in water, 61.7 g and 67 g/100 mL at 0°C and 100°C, respectively; soluble in sulfuric and nitric acids.Использование
Chromium(VI) oxide is widely used in chromium electroplating, and as protective coatings in corrosion/oxidation resistance of metals. It is also employed in CDs and DVDs. It is useful as an oxidant in Jones oxidation in acetic acid or acetone. It is used in the oxidation of primary alcohols into carboxylic acids, and secondary alcohols into ketones. With phosphoric acid, it is used as a stripping agent for anodic coatings of all types and in the production of syntheric rubies.Определение
chromium trioxide: A redcompound, CrO3; rhombic; r.d. 2.70;m.p. 196°C. It can be made by carefuladdition of concentrated sulphuricacid to an ice-cooled concentratedaqueous solution of sodium dichromatewith stirring. The mixture isthen filtered through sintered glass,washed with nitric acid, then dried at120°C in a desiccator.Chromium(VI) oxide is an extremelypowerful oxidizing agent,especially to organic matter; it immediatelyinflames ethanol. It is anacidic oxide and dissolves in water toform ‘chromic acid’, a powerful oxidizingagent and cleansing fluid forglassware. At 400°C, chromium(VI)oxide loses oxygen to givechromium(III) oxide.
Подготовка
Chromium(VI) oxide is prepared by heating sodium dichromate dihydrate with a slight excess of sulfuric acid in a steel tank or cast iron container: Na2Cr2O7 + 2H2SO4 → 2CrO3 + 2NaHSO4 + H2O The temperature of the mixture is kept above the melting point of chromium(VI) oxide to evaporate water and separate the top layer of sodium bisulfate from the molten chromium(VI) oxide at the bottom. Temperature control and duration of heating is very crucial in the process. Temperatures over 197°C (melting point), or allowing the molten mass to stand for a longer time, may result in decomposition of the product.Общее описание
Chromium(VI) oxide (CrO3) is a hexavalent chromium that is majorly used as an oxidizing agent. It oxidizes the C-H bonds in the aromatic rings to form benzoic acid from alkyl benzene. It can be prepared by adding concentrated sulphuric acid in potassium dichromate.Реакции воздуха и воды
Deliquescent. Water soluble, giving acidic solutions.Профиль реактивности
CHROMIUM TRIOXIDE is a powerful oxidizing agent. Can react violently upon contact with reducing reagents, including organic matter, leading to ignition or explosion. Dangerously reactive with acetone, alcohols, alkali metals (sodium, potassium), ammonia, arsenic, dimethylformamide, hydrogen sulfide, phosphorus, peroxyformic acid, pyridine, selenium, sulfur, and many other chemicals [Sax, 9th ed., 1996, p. 852]. Noncombustible but can accelerate the burning of combustible materials. Sufficient heat may be generated from the reaction with combustible materials to ignite the mass. Aqueous solutions corrode many metals rapidly. Often mixed with sulfuric acid to make "cleaning solution" for glass. Used cleaning solution in closed bottles may explode due to the build up of gaseous carbon dioxide arising from oxidation of organic impurities [Bryson, W. R., Chem. Brit., 1975, 11, p. 377].Угроза здоровью
Chromium(VI) oxide and other chromium(VI) salts are moderately toxic substances by ingestion; 1 to 15 g may be a fatal dose in humans. Ingestion of nonlethal doses of these compounds can cause stomach, liver, and kidney damage; symptoms may include clammy, cyanotic skin, sore throat, gastric burning, vomiting, and diarrhea. Chromic acid is irritating to the skin, and prolonged contact can cause ulceration. Inhalation of chromate dust or chromic acid mist can result in severe irritation of the nose, throat, bronchial tubes, and lungs and may cause coughing, labored breathing, and swelling of the larynx. Eye contact with chromium trioxide and its solutions can cause severe burns and possible loss of vision.Occupational exposure to chromium(VI) compounds has been related to an increased risk of lung cancer. Several hexavalent compounds of chromium, including chromium trioxide, are listed in IARC Group 1 ("carcinogenic to humans") and are classified as "select carcinogens" under the criteria of the OSHA Laboratory Standard. Long-term exposure to chromium trioxide or chromium(VI) salts may cause ulceration of the respiratory system and skin. Exposure to chromium trioxide by inhalation or skin contact may lead to sensitization. Chromium trioxide has exhibited teratogenic activity in animal tests.
Пожароопасность
These substances will accelerate burning when involved in a fire. May explode from heat or contamination. Some may burn rapidly. Some will react explosively with hydrocarbons (fuels). May ignite combustibles (wood, paper, oil, clothing, etc.). Containers may explode when heated. Runoff may create fire or explosion hazard.Воспламеняемость и взрывоопасность
Chromium(VI) oxide is not combustible but is a strong oxidizing agent and can accelerate the burning rate of combustible materials. Contact with easily oxidized organic or other combustible materials (including paper and oil) may result in ignition, violent combustion, or explosion. The use of dry chemical, carbon dioxide, Halon, or water spray extinguishers is recommended for fires involving chromium (VI) compounds.Возможный контакт
Chromium trioxide is used in plating and metal treatment, as a corrosion inhibitor; and as an oxidant; in aluminum anodizing, dye; ink, and paint manufacturing, tanning, engraving; and photography.хранилище
Chromium trioxide should be handled in a fume hood to avoid the inhalation of dust, and impermeable gloves should be worn at all times to prevent skin contact. The practice of using chromate solutions to clean glassware should be avoided. Chromium trioxide should be stored in areas separated from readily oxidized materials.Перевозки
UN1463 (anhydrous), Chromium trioxide, anhydrous, Chromium trioxide, anhydrous, Hazard Class: 5.1; Labels: 5.1-Oxidizer, 6.1-Poisonous materials, 8-Corrosive material. UN1755 (solution), Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
It separates when potassium or sodium dichromate are dissolved in conc H2SO4. Dry it in a vacuum desiccator over NaOH pellets. It is a hygroscopic, powerful oxidant and can ignite with organic compounds. It is a skin and pulmonary IRRITANT. [Keyes et al. Industrial Chemicals (Lowenheim & Moran eds.) 4th edn J. Wiley pp 270-274 1975.] CANCER SUSPECT.Несовместимости
Chromium trioxide is a strong oxidizer. The solution in water is a strong acid. Reacts violently with bases and is corrosive. Contact with reducing agents; fuels, organic chemicals, flammable and combustible materials, causing fire and explosion hazard. This chemical decomposes above 250C to chromic oxide and oxygen with increased fire hazard. Attacks metals in the presence of moisture.Утилизация отходов
Reduce to Cr(III). If material cannot be recovered and recycled, dispose of sludge in a chemical waste landfill.Хром(VI) оксид запасные части и сырье
сырьё
1of2
запасной предмет
- 2-Amino-6-nitropyridine
- 5-Nitropyridine-2-carboxylic acid
- 1-Инданон
- Хром (IV) оксид
- High temperature shift calalyst
- Цитронеллова кислота
- Флутопразепам
- adhesive J-22H
- Метиловый эфир 3-метилтиофен-2-карбоновой кислоты
- Метил 3-формилбензоа
- 6-NITROPYRIDINE-2-CARBOXYLIC ACID ETHYL ESTER
- carbinol synthesis catalyst
- Мурексид
- LEAD SILICOCHROMATE
- (R) - (+) - 2-Тетрагидрофурановая кислота
- 6-NITROPYRIDINE-2-CARBOXAMIDE
- Менадион
- DIPHENYLACETALDEHYDE
- 6-нитропиридин-2-карбоновой кислоты
- Norandrostenedione
- 2,5-Dibromobenzaldehyde
- Натрия бисульфат моногидра
- 6-NITROPYRIDINE-2-CARBONYL CHLORIDE
- Deprodone propionate
- 2,7-дибром-9-флуоренон
1of8
Хром(VI) оксид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 |