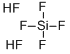
Hexafluorosilicic кислота
- английское имяHexafluorosilicic acid
- CAS №16961-83-4
- CBNumberCB3726895
- ФормулаF6H2Si
- мольный вес144.09
- EINECS241-034-8
- номер MDLMFCD00036289
- файл Mol16961-83-4.mol
| Температура кипения | 108-109°C |
| плотность | 1.22 g/mL at 20 °C (lit.) 1.31 g/mL at 25 °C |
| давление пара | 23hPa at 19.85℃ |
| показатель преломления | 1.3500 |
| Fp | 108-109°C |
| температура хранения | −20°C |
| растворимость | H2O: 1 mg/mL, clear, colorless |
| пка | 1.83[at 20 ℃] |
| форма | Liquid |
| цвет | Clear colorless |
| Удельный вес | 1.38 (40%) |
| Растворимость в воде | Miscible with water. |
| Гидролитическая чувствительность | 0: forms stable aqueous solutions |
| Мерк | 14,4182 |
| Пределы воздействия | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
| Стабильность | Stable in aqueous solution. |
| ИнЧИКей | AUJBMDCSBIPDEH-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 16961-83-4(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | FLUOSILICIC ACID |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 53V4OQG6U1 |
| Система регистрации веществ EPA | Fluosilicic acid (16961-83-4) |
| UNSPSC Code | 12352300 |
| NACRES | NA.23 |
| Коды опасности | C | |||||||||
| Заявления о рисках | 34-35-20/21/22 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-27 | |||||||||
| РИДАДР | UN 1778 8/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | VV8225000 | |||||||||
| F | 8-10 | |||||||||
| Примечание об опасности | Corrosive | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28111990 | |||||||||
| Банк данных об опасных веществах | 16961-83-4(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 oral in rat: 430mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H311:Токсично при попадании на кожу.
-
оператор предупредительных мер
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Hexafluorosilicic кислота химические свойства, назначение, производство
Описание
Hexafluorosilicic acid is a kind of inorganic acid. It is majorly used for the fluoridation of water in United State to minimize the incidence of dental caries and dental fluorosis. For chemical synthesis, it is majorly used for the manufacturing of aluminum fluoride and cryolite as well as many kinds of hexafluorosilicate salts. It can also be used for the production of silicon and silicon dioxide. It can also be used as an electrolyte in the Betts electrolytic process for refining lead. It is also a specialized reagent in organic synthesis for cleaving Si–O bonds of silyl ethers.Химические свойства
Fluosilicic acid,H2SiF6, also known as hydrofluorosilicic acid,is a colorless liquid that is soluble in water. It is highly corrosive and toxic,attacking glass and stoneware. Fluosilicic acid is used in water fluoridation, electroplating, and in manufacturing enamels and cement.Физические свойства
d 1.220 g cm?3 for a 25% aq solution.Использование
Hexafluorosilicic acid is commonly used as a source of fluoride. It is converted to a variety of useful hexafluorosilicate salts. It is also used as an electrolyte in the Betts electrolytic process for refining lead. It is an important organic reagent for cleaving Si-O bonds of silyl ethers. Further, it is used as wood a preservation agent and also used in surface modification of calcium carbonate.Общее описание
A colorless fuming liquid with a penetrating pungent odor. Corrosive to metals and tissue. Both the fumes and very short contact with the liquid can cause severe and painful burns. Used in water fluoridation, in hardening cement and ceramics, as a wood preservative.Реакции воздуха и воды
Fumes in air. Soluble in water with release of heat and corrosive fumes.Профиль реактивности
Hexafluorosilicic acid can react with strong acids (such as sulfuric acid) to release fumes of toxic hydrogen fluoride. Attacks glass and materials containing silica. Reacts exothermically with chemical bases (examples: amines, amides, inorganic hydroxides). Reacts with active metals, including iron and aluminum to dissolve the metal and liberate hydrogen and/or toxic gases. Can initiate polymerization in certain alkenes. Reacts with cyanide salts and compounds to release gaseous hydrogen cyanide. Flammable and/or toxic gases are also often generated by reactions with dithiocarbamates, isocyanates, mercaptans, nitrides, nitriles, sulfides, and weak or strong reducing agents. Additional gas-generating reactions may occur with sulfites, nitrites, thiosulfates (to give H2S and SO3), dithionites (SO2), and carbonates. Can catalyze (increase the rate of) chemical reactions. Decomposes when heated to the boiling point to produce very toxic and corrosive hydrogen fluoride gas.Опасность
Extremely corrosive by skin contact and inhalation.Угроза здоровью
Inhalation of vapor produces severe corrosive effect on mucous membrane. Ingestion causes severe burns of mouth and stomach. Contact with liquid or vapor causes severe burns of eyes and skin.Пожароопасность
Special Hazards of Combustion Products: Irritating fumes of hydrogen fluoride may form in fire.Промышленное использование
Hydrofluorosilicic acid (H2SiF6) is a colorless to light brown liquid. It is also manufactured from calcium fluoride or other fluoride-containing products. Hydrofluorosilic acid is a strong depressant for many silicates during flotation of a number of oxidic minerals. It is used for gangue depression during flotation of tin, columbite and tantalite.Профиль безопасности
Poison by subcutaneous route. A corrosive irritant to sktn, eyes, and mucous membranes. Will react with water or steam to produce toxic and corrosive fumes. When heated to decomposition it emits toxic fumes of F-. See also FLUORIDES.Возможный контакт
A solution of fluorosilicic acid is used for sterilization in the brewing and bottling industry, elec trolytic refining of lead; electroplating, hardening cement; removing mold, and others.Перевозки
UN1778 Fluorosilicic acid, Hazard class: 8; Labels: 8-Corrosive material.Несовместимости
The aqueous solution is a strong acid. Reacts with water or steam to produce toxic and corrosive fumes of hydrogen fluoride. Incompatible, and may react violently with: bases, aliphatic amines; alkanolamines, alkylene oxides; aromatic amines; amides, ammonia, ammonium hydroxide; calcium oxide; epichlorohydrin, iso cyanates, oleum, organic anhydrides; sulfuric acid; strong oxidizers; vinyl acetate; water. Attacks glass, concrete, and ceramics. The anhydrous form dissociates almost instantly into silicon tetrafluoride and hydrogen fluoride.Утилизация отходов
Add slowly to a large amount of soda ash in solution. Discharge to sewer with large volumes of waterиспользованная литература
Robinson, Tim. "Innovative Processes in Electrometallurgy." Innovative Process Development in Metallurgical Industry. Springer International Publishing, 2016. 385- 392.Sarawade, Pradip B., et al. "Recovery of high surface area mesoporous silica from waste hexafluorosilicic acid (H 2 SiF 6) of fertilizer industry." Journal of hazardous materials 173.1 (2010): 576-580.
Kauffman, Joel M. "Water fluoridation: a review of recent research and actions." Journal of American Physicians and Surgeons 10.2 (2005): 38.
Krot, V. V., et al. "ChemInform Abstract: Preparation of Amorphous Silicon Dioxide from Hexafluorosilicic Acid." Cheminform 23.48(1992):no-no.
Zorya, L., and V. Krot. "Method of high-purity silica production from hexafluorosilicic acid." Reaction Kinetics & Catalysis Letters 50.1-2(1993):349-354.
Hexafluorosilicic кислота запасные части и сырье
Hexafluorosilicic кислота поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 |