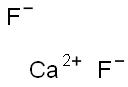
Фторид кальция
- английское имяCalcium fluoride
- CAS №7789-75-5
- CBNumberCB5225741
- ФормулаCaF2
- мольный вес78.07
- EINECS232-188-7
- номер MDLMFCD00010907
- файл Mol7789-75-5.mol
| Температура плавления | 1402 °C | ||||||||||||||
| Температура кипения | 2500 °C (lit.) | ||||||||||||||
| плотность | 3.18 g/mL at 25 °C (lit.) | ||||||||||||||
| показатель преломления | 1.434 | ||||||||||||||
| Fp | 2500°C | ||||||||||||||
| температура хранения | -20°C | ||||||||||||||
| растворимость | Slightly soluble in acid; insoluble in acetone. | ||||||||||||||
| форма | rod | ||||||||||||||
| Удельный вес | 3.18 | ||||||||||||||
| цвет | white | ||||||||||||||
| Запах | Odorless | ||||||||||||||
| РН | 4 (20°C, 100g/L in H2O, slurry) | ||||||||||||||
| Биологические источники | rabbit | ||||||||||||||
| Растворимость в воде | 0.015 g. per 100g H20 at room temperature.(INSOLUBLE) | ||||||||||||||
| Чувствительный | Hygroscopic | ||||||||||||||
| Кристальная структура | CaF2 type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Мерк | 14,1667 | ||||||||||||||
| Константа произведения растворимости (Ksp) | pKsp: 8.28 | ||||||||||||||
| Space group | Fm3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Пределы воздействия | ACGIH: TWA 2.5 mg/m3 NIOSH: IDLH 250 mg/m3; TWA 2.5 mg/m3 |
||||||||||||||
| Диэлектрическая постоянная | 2.5(Ambient) | ||||||||||||||
| Стабильность | Stable. Calcium fluoride is inert to organic chemicals and many acids, including HF. It will slowly dissolve in nitric acid. | ||||||||||||||
| ИнЧИКей | WUKWITHWXAAZEY-UHFFFAOYSA-L | ||||||||||||||
| Справочник по базе данных CAS | 7789-75-5(CAS DataBase Reference) | ||||||||||||||
| Рейтинг продуктов питания EWG | 1-2 | ||||||||||||||
| FDA UNII | O3B55K4YKI | ||||||||||||||
| Справочник по химии NIST | Calcium fluoride(7789-75-5) | ||||||||||||||
| Система регистрации веществ EPA | Calcium difluoride (7789-75-5) | ||||||||||||||
| UNSPSC Code | 41116107 | ||||||||||||||
| NACRES | NA.41 |
| Коды опасности | Xi,Xn | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-37/39-36 | |||||||||
| РИДАДР | 3288 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | EW1760000 | |||||||||
| Примечание об опасности | Irritant | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28261900 | |||||||||
| Банк данных об опасных веществах | 7789-75-5(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 i.p. in mice: 2638.27 mg/kg (Stratmann) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P405:Хранить в недоступном для посторонних месте.
Фторид кальция химические свойства, назначение, производство
Химические свойства
Calcium fluoride, CaF2, also known as fluorite and feldspar, is a colorless solid composed of cubic crystals. It has a low water solubility, but is readily soluble in ammonium salt solutions. Calcium fluoride is used in the synthesis of hydrofluoric acid and in etching glass.Calcium Fluoride is not hygroscopic and is relatively inert. It has a sufficiently high resistance to thermal and mechanical shock, and it is easily fabricated into a variety of detector geometries.
Calcium Fluoride has a very low vapor pressure, allowing it to be used in a wide variety of vacuum applications. It is insoluble in water and most organic solvents, permitting radioactive samples in solution to be placed in direct contact with the crystal.
Calcium Fluoride is a transparent material used for detecting γ-rays up to several hundred keV and for detecting charged particles. Because of its low atomic number, the material’s photofraction is relatively small which limits its use in γ-ray spectrometry at higher energies. However, the low atomic number makes Calcium Fluoride an ideal material for the detection of β-particles because of the small amount of backscattering.
Физические свойства
White cubic crystal or powder; refractive index 1.434; density 3.18 g/cm3; hardness 4 Mohs; melts at 1,418°C; vaporizes at 2,533°C; insoluble in water (16 mg/L at 20°C); Ksp 3.9x10-11; slightly soluble in dilute mineral acid; soluble in concentrated acids (with reaction).Использование
Fluorspar is the main primary source of fluorine and its Compounds. In ferrous metallurgy it is used as a flux to increase the fluidity of the slag. The steel industry is the largest consumer; the chemical industry, second and glass and ceramics, third. Synthetic fluorspar is used in the optical industry (transmits u.v. rays), and pure calcium fluoride is used as catalyst in dehydration and dehydrogenations. Used to fluoridate drinking water.Методы производства
The commercial product is obtained from naturally occurring mineral fluorspar, which is purified and powdered. Also, it may be precipitated by mixing a solution of sodium fluoride with a soluble calcium salt:Ca(NO3)2 + 2NaF → CaF2 + NaNO3
Alternatively, it may be obtained by treating calcium carbonate with hydrofluoric acid:CaCO3 + 2HF → CaF2 + CO2 + H2O.
Определение
A pure naturally occurring form of calcium fluoride.Общее описание
Odorless gray powder or granules. Sinks in water.Профиль реактивности
Calcium fluoride has weak oxidizing or reducing powers. Redox reactions can however still occur. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive.Опасность
An irritant.Угроза здоровью
Little acute toxicityПромышленное использование
Also called fluorite, fluorspar is a crystalline or massive granular mineral of the composition CaF2, used as a flux in the making of steel, for making hydrofluoric acid, in opalescent glass, in ceramic enamels, for snaking artificial cryolite, as a binder for vitreous abrasive wheels,and in the production of white cement. It is a better flux for steel than limestone, making a fluid slag, and freeing the iron of sulfur and phosphorus.Acid spar is a grade used in making hydrofluoric acid. It is also used for making refrigerants, plastics, and chemicals, and for aluminum reduction. Optical fluorspar is the highest grade but is not common. Fluoride crystals for optical lenses are grown artificially from acid-grade fluorspar. Pure calcium fluoride, Ca2F6, is a colorless crystalline powder used for etching glass, in enamels, and for reducing friction in machine bearings. It is also used for ceramic parts resistant to hydrofluoric acid and most other acids. Calcium fluorite has silicon in the molecule and is a crystalline powder used for enamels. The clear rhombic fluoride crystals used for transforming electric energy into light are lead fluoride, PbF2.
Профиль безопасности
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. An experimental teratogen. Other experimental reproductive effects. Mutation data reported. See also FLUORIDES and CALCIUM COMPOUNDS. When heated to decomposition it emits toxic fumes of F-.Возможный контакт
Calcium fluoride is used for production of hydrofluoric acid; as a flux in steel manufacture; in smelting; electric arc welding, making glass and ceramics; and to fluoridate drinking water.Перевозки
Calcium fluoride is not specifically covered by DOT in its Performance-Oriented Packaging Standards.Несовместимости
Dust may form explosive mixture with air. Reacts with water, moist air, and steam, releasing flammable hydrogen gas; and may self-ignite in air. A strong reducing agent; incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Incompatible with metal halogenates, silver fluoride, and tetrahydrofuranФторид кальция запасные части и сырье
сырьё
1of2
запасной предмет
- Цианамид кальция
- КРИОЛИТ
- Hexafluorosilicic кислота
- MAGNESIUM HEXAFLUOROACETYLACETONATE DIHYDRATE
- Potassium dihydrogen orthophosphate
1of3
Фторид кальция поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 2039322489 | United States | 168 | 58 | ||
| +86-0314-6276212 +8618631452027 |
China | 27 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-17736087130 +86-18633844644 |
China | 994 | 58 |
Фторид кальция Обзор)
1of4