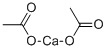
Кальций уксуснокислый
- английское имяCalcium acetate
- CAS №62-54-4
- CBNumberCB8312095
- ФормулаC4H6CaO4
- мольный вес158.17
- EINECS200-540-9
- номер MDLMFCD00012448
- файл Mol62-54-4.mol
| Температура плавления | 160°C (dec.) |
| плотность | 1,5 g/cm3 |
| показатель преломления | 1.5500 |
| FEMA | 2228 | CALCIUM ACETATE |
| Fp | 160°C |
| температура хранения | Hygroscopic, Room Temperature, under inert atmosphere |
| растворимость | H2O: 1 M at 20 °C, clear, colorless |
| форма | Powder |
| Удельный вес | 1.50 |
| цвет | white |
| РН | 8(1 mM solution);8.43(10 mM solution);8.77(100 mM solution);9.13(1000 mM solution) |
| Запах | mild acetic |
| Odor Type | acetic |
| Растворимость в воде | soluble |
| Разложение | 160 ºC |
| Стабильность | Stable. Non-flammable. Incompatible with strong oxidizing agents. |
| ИнЧИКей | VSGNNIFQASZAOI-UHFFFAOYSA-L |
| LogP | -0.29 |
| Справочник по базе данных CAS | 62-54-4(CAS DataBase Reference) |
| FDA 21 CFR | 182.6197; 184.1185; 582.6185; 582.6197; 175.300; 181.29 |
| Вещества, добавляемые в пищу (ранее EAFUS) | CALCIUM ACETATE |
| Специальный комитет по веществам GRAS | Calcium acetate |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | Y882YXF34X |
| Код УВД | V03AE07 |
| Система регистрации веществ EPA | Calcium acetate (62-54-4) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 26-36 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | AF7525000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29152990 | |||||||||
| Банк данных об опасных веществах | 62-54-4(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P332+P313:При возникновении раздражения кожи: обратиться за медицинской помощью.
Кальций уксуснокислый химические свойства, назначение, производство
Описание
Calcium acetate is a chemical compound which is calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous form is very hygroscopic; therefore the monohydrate (Ca(CH3COO)2?H2O) is the common form.Химические свойства
Calcium acetate occurs as a white or almost white, odorless or almost odorless, hygroscopic powder.Использование
Calcium Acetate is the calcium salt of acetic acid which functions as a sequestrant and mold control agent. it contains approximately 25% calcium. it is a white odorless powder which is readily soluble in water with a solubility of approximately 37 g in 100 g water at 0°c. its solubility decreases with increasing temperature, with a sol- ubility of approximately 29 g in 100 g of water at 100°c.Определение
ChEBI: The calcium salt of acetic acid. It is used, commonly as a hydrate, to treat hyperphosphataemia (excess phosphate in the blood) in patients with kidney disease: the calcium ion combines with dietary phosphate to form (insoluble) calcium phosphate, which is excreted in the faeces.Методы производства
Calcium acetate can be prepared by soaking calcium carbonate (found in eggshells, or in common carbonate rocks such as lime stone or marble) in vinegar:CaCO3 + 2CH3COOH → Ca(CH3COO)2 + H2O + CO2
Since both reagents would have been available pre-historically, the chemical would have been observable as crystals then.
Подготовка
Produced by calcium hydroxide neutralization of acetic acid.Общее описание
Calcium Acetate belongs to the group of calcium salts, widely used as phosphorus binders in patients with chronic renal failure.Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Фармацевтические приложения
Calcium acetate is used as a preservative in oral and topical formulations.Therapeutically, parenteral calcium acetate acts as a source of calcium ions for hypocalcemia or electrolyte balance. Oral calcium acetate is used as a complexing agent for hyperphosphatemia in dialysis patients. Calcium acetate is also used in the food industry as a stabilizer, buffer and sequestrant.
Профиль безопасности
Poison by intravenous and intraperitoneal routes. Mutation data reported. See also CALCIUM COMPOUNDS. When heated to decomposition it emits acrid smoke and fumes.Безопасность
Calcium acetate is used in oral and topical formulations. The pure form of calcium acetate is toxic by IP and IV routes.LD50 (mouse, IP): 0.075 g/kg
LD50 (mouse, IV): 0.052 g/kg
LD50 (rat, oral): 4.28 g/kg
хранилище
Calcium acetate is stable although very hygroscopic, and so the monohydrate is the common form. It decomposes on heating (above 1608℃) to form calcium carbonate and acetone.Store in well-closed airtight containers.
Методы очистки
Recrystallise it from water (3mL/g) by partial evaporation in a desiccator. [Beilstein 2 IV 113.]Несовместимости
Calcium acetate is incompatible with strong oxidizing agents and moisture.Регуляторный статус
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (oral suspensions and tablets; topical emulsions, lotions, and creams). Included in nonparenteral medicines (oral tablets) licensed in the UK.Кальций уксуснокислый запасные части и сырье
сырьё
1of3
запасной предмет
1of2
Кальций уксуснокислый поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +16837774622 | China | 295 | 58 | |
| +86-18353166132 +86-18353166132 |
China | 983 | 58 | |
| +8618092446649 | China | 1143 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +8615373025980 | China | 895 | 58 | |
| +8618805133257 | China | 132 | 60 |
Кальций уксуснокислый Обзор)
1of4