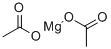
Ацетат магния
- английское имяMagnesium acetate
- CAS №142-72-3
- CBNumberCB2160485
- ФормулаC4H6MgO4
- мольный вес142.39
- EINECS205-554-9
- номер MDLMFCD00003539
- файл Mol142-72-3.mol
| Температура плавления | 72-75 °C(lit.) |
| плотность | 1.5000 |
| показатель преломления | n |
| растворимость | H2O: 1 M at 20 °C, clear, colorless |
| пка | 4.756[at 20 ℃] |
| форма | Liquid |
| цвет | white orthorhombic/monoclinic crystals, crystalline |
| РН | 7.95(1 mM solution);8.23(10 mM solution);8.41(100 mM solution);8.78(1000 mM solution) |
| Растворимость в воде | Not miscible or difficult to mix with water. |
| λмакс | λ: 260 nm Amax: 0.04 λ: 280 nm Amax: 0.02 |
| Мерк | 13,5676 |
| БРН | 3692530 |
| Стабильность | Stable. Incompatible with strong oxidizing agents. Avoid moisture. |
| ИнЧИКей | UEGPKNKPLBYCNK-UHFFFAOYSA-L |
| LogP | -1.38 |
| Справочник по базе данных CAS | 142-72-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 0E95JZY48K |
| Справочник по химии NIST | Magnesium acetate(142-72-3) |
| Система регистрации веществ EPA | Magnesium acetate (142-72-3) |
| UNSPSC Code | 12161700 |
| NACRES | NA.25 |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H226:Воспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H332:Вредно при вдыхании.
H312:Вредно при попадании на кожу.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P312:Обратиться за медицинской помощью при плохом самочувствии.
P363:Перед повторным использованием выстирать загрязненную одежду.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Ацетат магния химические свойства, назначение, производство
Описание
Anhydrous Magnesium Acetate has the chemical formula Mg(CH3COO)2 and in its hydrous form, Magnesium Acetate Tetrahydrate, it has the chemical formula Mg(CH3COO)2.4H2O. In this compound the magnesium metal has an oxidation state of 2+.
Magnesium acetate is the magnesium salt of acetic acid.It is deliquescent and upon heating, it decomposes to form magnesium oxide.Magnesium acetate is commonly used as a source of magnesium or as a chemical reagent.
Химические свойства
white deliquescent crystalsФизические свойства
Magnesium acetate appears as white hygroscopic crystals. It smells like acetic acid and is soluble in water. When it is in an aqueous solution its pH can be considered to be neutral.Характеристики
In 1881 Charles Clamond invented the Clamond basket, one of the first effective gas mantles. The reagents used in this invention included magnesium acetate, magnesium hydroxide and water.Magnesium acetate is commonly used as a source of magnesium or for the acetate ion in chemistry experiments. One example of this is when magnesium acetate and magnesium nitrate were both used to perform molecular dynamics simulations and surface tension measurements. In the experiment the authors found that the acetate had a stronger affinity for the surface compared to the nitrate ion and that the Mg2+ strongly repelled away from the air/liquid interference. They also found that the Mg2+ had a stronger tendency to bind with the acetate ion compared to the nitrate.
One of the more prevalent uses of magnesium acetate is in the mixture called calcium magnesium acetate (CMA). It is a mixture of calcium acetate and magnesium acetate. CMA is thought of as an environmentally friendly alternative deicer to NaCl and CaCl2. CMA also acts as a powerful SO2, NOx, and toxic particulate emission control agent in coal combustion processes to reduce acid rain, and as an effective catalyst for the facilitation of coal combustion.
Использование
Dye fixative in textile printing, deodorant, disinfectant, and antiseptic.Magnesium acetate is used in chemistry and molecular biology as a reagent and a source of magnesium. Magnesium has a variety of biological roles in enzymology, cell membrane and wall structural integrity, muscle cell physiology, and nucleic acid structure.Magnesium is an essential co-factor in many enzymes, including deoxyribonuclease (DNase), the restriction enzymes EcoR I and EcoR V, and Ribonuclease H. Magnesium also stabilizes polymeric nucleic acids such as transfer RNA and ribozymes.
Magnesium acetate has been widely used in the crystallization of proteins. A protocol for the separation of MB and BB isoenzymes of creatine kinase and the LD1 isoenzyme of lactate dehydrogenase that involves elution with magnesium acetate has been published.
Определение
ChEBI: The magnesium salt of acetic acid.Безопасность
Magnesium Acetate is a relatively safe compound to handle and has been given a health hazard rating of zero. However, it should always be handled with gloves and safety goggles. If it is gets in the eyes, the skin, ingested, or inhaled it will cause irritation in the respective areas: eyes, skin, gastrointestinal system, and lungs.хранилище
Due to the fact that it is very hygroscopic, it must be stored away from water. It is also incompatible with strong oxidizers and should not be mixed with them.Методы очистки
Crystallise it from anhydrous acetic acid, then dry it under vacuum for 24hours at 100o. [Nencollas J Chem Soc 744 1956, Beilstein 2 IV 113.]Ацетат магния запасные части и сырье
Ацетат магния поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +8619931165850 | China | 1000 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | |
| +8615373025980 | China | 895 | 58 | |
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 |