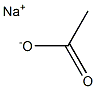
АЦЕТАТ НАТРИЯ
- английское имяSodium acetate
- CAS №127-09-3
- CBNumberCB1230044
- ФормулаC2H3NaO2
- мольный вес82.03379
- EINECS204-823-8
- номер MDLMFCD00012459
- файл Mol127-09-3.mol
| Температура плавления | >300 °C (dec.)(lit.) |
| плотность | 1.01 g/mL at 20 °C |
| FEMA | 3024 | SODIUM ACETATE |
| показатель преломления | 1.4640 |
| Fp | >250 °C |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | H2O: 3 M at 20 °C, clear, colorless |
| форма | powder |
| пка | 4.756[at 20 ℃] |
| цвет | white |
| Удельный вес | 1.45 |
| Запах | Slight acetic acid |
| Водородный показатель | 8.5 - 9.9 at 246 g/l at 25 °C |
| РН | 7.87(1 mM solution);8.33(10 mM solution);8.75(100 mM solution);9.04(1000 mM solution) |
| Odor Type | odorless |
| Биологические источники | synthetic |
| Растворимость в воде | 500 g/L (20 ºC) |
| Чувствительный | Hygroscopic |
| Гидролитическая чувствительность | 0: forms stable aqueous solutions |
| λмакс | λ: 260 nm Amax: 0.03 λ: 280 nm Amax: 0.02 |
| Мерк | 14,8571 |
| БРН | 3595639 |
| Температура кипения | >400°C(decomposition) |
| Стабильность | Stable. Incompatible with strong oxidizing agents, halogens. Moisture sensitive. |
| ИнЧИКей | VMHLLURERBWHNL-UHFFFAOYSA-M |
| LogP | -3.72 |
| FDA 21 CFR | 184.1721; 582.1721; 173.310; 182.70 |
| Справочник по базе данных CAS | 127-09-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | NVG71ZZ7P0 |
| Код УВД | B05XA08 |
| Справочник по химии NIST | Sodium ethanoate(127-09-3) |
| Система регистрации веществ EPA | Sodium acetate (127-09-3) |
| Поглощение | ≤0.01 at 260nm in H2O at 100mM ≤0.01 at 280nm in H2O at 100mM |
| UNSPSC Code | 51191566 |
| NACRES | NA.55 |
| Заявления о безопасности | 22-24/25 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | AJ4300010 | |||||||||
| F | 3 | |||||||||
| Температура самовоспламенения | 607 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29152200 | |||||||||
| Банк данных об опасных веществах | 127-09-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 3530 mg/kg LD50 dermal Rabbit > 10000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
АЦЕТАТ НАТРИЯ химические свойства, назначение, производство
Описание
Sodium acetate (CH3COONa) is the sodium salt of acetic acid. It appears as a colorless deliquescent salt with a wide range of applications. In industry, it can be used in textile industry to neutralize sulfuric acid waste streams and as a photoresist upon using aniline dyes. In concrete industry, it can be used as a concrete sealant to mitigate the water damage. In food, it can be used as a seasoning. It can also be used as a buffer solution in lab. In addition, it is also used in heating pads, hand warmers and hot ice. For laboratory use, it can be produced by the reaction between acetate with the sodium carbonate, sodium bicarbonate and sodium hydroxide. In industry, it is prepared from the glacial acetic acid and sodium hydroxide.Химические свойства
Sodium acetate, CH3COONa, also abbreviated NaOAc , also sodium ethanoate, is the sodium salt of acetic acid, was made by the reaction of acetic acid with sodium carbonate. It is soluble in water but less so in alcohol. This colourless salt has a wide range of uses. Sodium acetate was used as a pH modifier for toning baths.Физические свойства
Anhydrous salt is a colorless crystalline solid; density 1.528 g/cm3; melts at 324°C; very soluble in water; moderately soluble in ethanol. The colorless crystalline trihydrate has a density 1.45 g/cm3; decomposes at 58°C; is very soluble in water; pH of 0.1M aqueous solution is 8.9; moderately soluble in ethanol, 5.3 g/100mL.Вхождение
Acetic acid or acetates are present in most plant and animal tissues in small, but detectable amountsИспользование
Used as buffers.Acidity regulation (buffering)
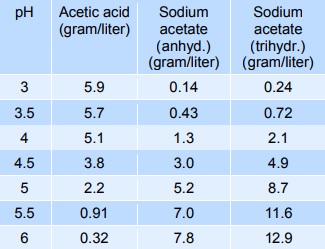
Sodium acetate mixed with acetic acid forms a pH buffer, which can be used to stabilise the pH of foods in the pH-range from 3 to 6. The table below gives indicative values of the composition needed to give a certain pH. The mixtures below can be diluted at least 10 times with minimum effect on pH, however, the stability decreases.
Подготовка
Sodium acetate is prepared by reacting sodium hydroxide or sodium carbonate with acetic acid in aqueous solution. The solution is evaporated to obtain hydrated crystals of sodium acetate.NaOH + CH3COOH → CH3COONa + H2O
Na2CO3 + CH3COOH → 2CH3COONa + CO2 + H2O
Определение
ChEBI: Sodium acetate is an organic sodium salt. It contains an acetate.Синтез
For laboratory use, sodium acetate is very inexpensive, and is usually purchased instead of being synthesized. It is sometimes produced in a laboratory experiment by the reaction of acetic acid (ethanoic acid) with sodium carbonate, sodium bicarbonate, or sodium hydroxide. These reactions produce aqueous sodium acetate and water. Carbon dioxide is produced in the reaction with sodium carbonate and bicarbonate, and it leaves the reaction vessel as a gas (unless the reaction vessel is pressurized). This is the well-known "volcano" reaction between baking soda (sodium bicarbonate) and vinegar.CH3COOH + NaHCO3 → CH3COONa + H2O + CO2
Industrially, sodium acetate is prepared from glacial acetic acid and sodium hydroxide.
CH3COOH + NaOH → CH3COONa + H2O.
Реакции
Sodium acetate can be used to form an ester with an alkyl halide such as bromo ethane:CH3COONa + Br CH2CH3→ CH3COOCH2CH3+ NaBr
Caesium salts catalyze this reaction.
Общее описание
Sodium Acetate is reported to inhibit the growth of Listeria monocytogenes.Профиль реактивности
When sodium acetate reacts with strong acids, irritating, noxious vapors of acetic acid are usually produced. Sodium acetate is sufficiently basic to catalyze the violent polymerization of diketene, perhaps as well as other reactive dimers that are susceptible to polymerization in the presence of a mild base.Биологическая активность
Commonly used laboratory reagentПрофиль безопасности
Poison by intravenous route. Moderately toxic by ingestion. A skin and eye irritant. Migrates to food from packagmg materials. Violent reaction with F2, m03, diketene. When heated to decomposition it emits toxic fumes of Na2O.Описание
Ацетат натрия хорошо растворяется в воде; плохо растворяется в спирте, эфире, гигроскопичен. Тпл 324°С (безводный). Природный источник- во всех живых клетках как продукт расщепления жиров и углеводов.
Химические свойства
Белый, кристаллический порошок, со слабым запахом уксусной кислоты, гигроскопичен.
Безопасность
Ацетат натрия способствует синтезу жира в преджелудках, образованию и секреции желчных кислот, усиливает всасывание жиров из кишечника, активизирует синтез аминокислот и плазменных белков. Наличие в препарате иона натрия стимулирует функцию печени, почек, слизистой оболочки кишечника, улучшает электролитный баланс и регулирует кислотно-щелочное равновесие организма.
Производство
Ацетат натрия получают взаимодействием карбоната натрия или едкого натра с уксусной кислотой или ее эфирами, при сухой перегонке древесины с карбонатом натрия и др.
Методы очистки
Crystallise it from acetic acid and keep it under vacuum for 10hours at 120o. Alternatively, it is crystallised from aqueous EtOH, as the trihydrate. This material can be converted to anhydrous salt by heating slowly in a porcelain, nickel or iron dish, so that the salt liquefies. Steam is evolved and the mass again solidifies. Heating is now increased so that the salt melts again. (NB: if it is heated too strongly, the salt can char; avoid this.) After several minutes, the salt is allowed to solidify and is cooled to a convenient temperature (in a desiccator) before being powdered and bottled. The water content should now be less than 0.02%. [Beilstein 2 II 113, 2 III 184, 2 IV 109.]АЦЕТАТ НАТРИЯ запасные части и сырье
сырьё
запасной предмет
- Ибудиласт
- 7H-DIBENZO[C,G]CARBAZOLE
- 4-METHYL-2-METHYLSULFANYL-PYRIMIDINE-5-CARBOXYLIC ACID ETHYL ESTER
- Дегидрохолевая кислота
- CHEMBRDG-BB 4023079
- ethyl 2-(methylsulfanyl)-4-(trifluoromethyl)pyrimidine-5-carboxylate
- 2 - (метилтио) этанол
- Пиридазин-3-карбоновой кислоты
- Citronellyl acetate
- ^-Terpinyl ацета
- 3-(3-Nitrophenyl)propionic acid
- эндо-8-изопропил-8-азабицикло[3.2.1]октан-3-ол
- 5-НИТРО-2-ФУРОНИТРИЛ
- 6-Azathymine
- Cyclohexanol, 5-methyl-2-(1-methylethyl)-, acetate, (1R,2S,5R)-rel-
- Натрий диацетат
- 2-Nitrodiphenylamine
- 4-Aminotetrahydropyran hydrochloride
- Cinnamyl acetate
- 3-NITRO-PYRIDINE-2-CARBOXYLIC ACID
- 3-ацетил-4-гидрокси-2-бензопирон
- CYCLOOCTENE OXIDE
- Ингибитор трипсина
- Левосимендан
- КИСЛОТНЫЙ СИНИЙ 92
1of8
АЦЕТАТ НАТРИЯ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618805133257 | China | 132 | 60 | ||
| +862156762820 +86-13564624040 |
China | 7707 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +1-+1(833)-552-7181 | United States | 57505 | 58 | ||
| +86-18532138899 +86-18532138899 |
China | 939 | 58 | ||
| +8613817748580 | China | 40066 | 58 | ||
| +8615175982296 | China | 33 | 58 | ||
| +86-29-81148696 +86-15536356810 |
China | 3882 | 58 | ||
| +8613223293093 | China | 80 | 58 |