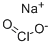
Натрий хлорит
- английское имяSodium chlorite
- CAS №7758-19-2
- CBNumberCB8854304
- ФормулаClNaO2
- мольный вес90.44
- EINECS231-836-6
- номер MDLMFCD00003478
- файл Mol7758-19-2.mol
| Температура плавления | 190 °C (dec.) |
| Температура кипения | ≥100°C/1013hPa |
| плотность | 2.5 g/cm3 |
| давление пара | 0Pa at 25℃ |
| температура хранения | 2-30°C |
| растворимость | Methanol (Slightly), Water (Slightly) |
| форма | Powder |
| цвет | White |
| Запах | odorless |
| Водородный показатель | 10 - 11 |
| РН | 12-13 (20°C in H2O) |
| Пределы взрываемости | 7% |
| Растворимость в воде | 39 g/100 mL (17 ºC) |
| Чувствительный | Hygroscopic |
| Мерк | 14,8600 |
| Диэлектрическая постоянная | 6.1(Ambient) |
| Стабильность | Stable. Incompatible with phosphorus, sulphur, zinc, ammonia, finely powdered metals, strong reducing agents, acids, organic materials. |
| Стандарт первичной питьевой воды EPA | MCL:1,MCLG:0.8 |
| LogP | -2.7 at 25℃ |
| Справочник по базе данных CAS | 7758-19-2(CAS DataBase Reference) |
| FDA 21 CFR | 186.1750; 165.110; 175.105 |
| Вещества, добавляемые в пищу (ранее EAFUS) | SODIUM CHLORITE |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | G538EBV4VF |
| Код УВД | D03AX11 |
| МАИР | 3 (Vol. 52) 1991 |
| Система регистрации веществ EPA | Sodium chlorite (7758-19-2) |
| Пестициды Закон о свободе информации (FOIA) | Sodium chlorite |
| UNSPSC Code | 12352302 |
| NACRES | NA.55 |
| Коды опасности | O,Xn,T+,T,N | |||||||||
| Заявления о рисках | 8-22-24-32-34-9-26-25-14-36/37/38-21-50 | |||||||||
| Заявления о безопасности | 17-26-36/37/39-45-50A-38-36-61-28 | |||||||||
| РИДАДР | UN 2813 4.3/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | VZ4800000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 5.1 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28289000 | |||||||||
| Банк данных об опасных веществах | 7758-19-2(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)





-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H400:Чрезвычайно токсично для водных организмов.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H310:Смертельно при попадании на кожу.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H271:Сильный окислитель; может вызвать возгорание или взрыв.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Натрий хлорит химические свойства, назначение, производство
Химические свойства
It is a white crystalline powder or flakes that is readily soluble in water. It is slightly hygroscopic.Использование
Sodium chlorite is used in the in situ generation of chlorine dioxide for stripping of textiles, bleaching, pulp and paper industries. It acts as a disinfectant in water treatment plant and as a preservative in eye drops. It is also used in contact lens cleaning solution and for sanitizing air ducts. It is associated with zinc chloride and used as a component in therapeutic rinses, toothpastes, mouth sprays and gels. It is utilized for the synthesis of 4-oxo-2-alkenoic acids from alkyl furans. Further, it is involved as a reagent in Pinnick oxidation reaction to prepare carboxylic acid from aldehydes.Определение
ChEBI: Sodium chlorite is an inorganic sodium salt in which chlorite is the counterion. It has a role as an oxidising agent. It is an inorganic sodium salt and a chlorite salt.Общее описание
Sodium chlorite appears as a white crystalline solid. Difficult to burn, but accelerates the burning of organic substances. Forms explosive mixtures with certain combustible materials. May explode under prolonged exposure to heat or fire. Used in water purification, to bleach wood pulp, textile, fats, oils; and for many other uses.Реакции воздуха и воды
Soluble in water.Профиль реактивности
SODIUM CHLORITE SOLUTION is an oxidizing agent. Can react with acids to form spontaneously explosive chlorine dioxide gas (ClO2). Reacts with ammonia to produce ammonium chlorite, which is shock-sensitive. Finely divided metallic or organic substances in dry mixture with chlorites are highly flammable and may be ignited on friction (Lab. Gov. Chemist 1965). A mixture of organic matter and solid sodium chlorite can be extremely sensitive to heat, impact, or friction (Diox Process 1949). Sodium chlorite reacts very violently with organic materials containing divalent sulfur or with free sulfur (may ignite).Опасность
Flammable, strong oxidizing agent, dan- gerous fire and moderate explosion risk. (Solution) Strong irritant to skin and tissue.Угроза здоровью
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Сельскохозяйственное использование
Chlorite is a group of greenish clay minerals of variable composition (similar to mica in structure), which crystallizes in the monoclinic system. The term chlorite is derived from 'chloros', the Greek word for green.Chlorites are composed of complex silicates of aluminum, magnesium and iron in combination with water.
These are often called 2:2 type clays because they are similar to the unit lattice of vermiculite. But strictly speaking, they are 2:1:1 type clays. A layer of chlorite has 2 silicate tetrahedral units, one alumina octahedral unit and one magnesium octahedral sheet. It has a low cation exchange capacity. Chlorites are most commonly found in low-grade metamorphic rocks. They also occur as secondary minerals in igneous rocks as alteration products of pyroxenes, amphiboles and micas.
Chlorites are infrequent in soils and when present, make up a small fraction of clay minerals. Chlorites are primary minerals and form vermiculites and smectites. Chlorites do not swell on wetting.
Профиль безопасности
Poison by ingestion. An experimental teratogen. Experimental reproductive effects. Questionable carcinogen with experimental carcinogenic data. Mutation data reported. May act as an irritant due to its oxidizing power. A powerful oxidzing agent; ignited by friction, heat, or shock. An explosive sensitive to impact or heating to 200'. Potentially explosive reaction with acids, oils, organic matter, oxahc acid + water, zinc. Violent reaction or iption with carbon (above 60'), ethylene glycol (at loo'), phosphorus (above SO0), sodum dithionate, sulfur-containing materials. Can react vigorously on contact with reducing materials. When heated to decomposition it emits highly toxic fumes of Cland NazO. Used as a bleachmg agent. See also CHLORITES.Методы очистки
Crystallise the chlorite from hot water and store it in a cool place. It has also been crystallised from MeOH by counter-current extraction with liquid ammonia [Curti & Locchi Anal Chem 29 534 1957]. A major impurity is chloride ion which can be removed by recrystallisation from 0.001M NaOH. [Schmeisser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 312 1963.]Натрий хлорит запасные части и сырье
Натрий хлорит поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-15662691337 +86-15662695772 |
China | 998 | 58 | ||
| +8613817748580 | China | 40066 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-86-4001020630 +8619831957301 |
China | 1000 | 58 | ||
| 0086-571-86990109 | China | 104 | 55 | ||
| +86-86-02137122233 +8613795318958 |
China | 299 | 55 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 86-22-66880623 +8618622897568 |
China | 571 | 58 | ||
| +86 18953170293 | China | 2930 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 |