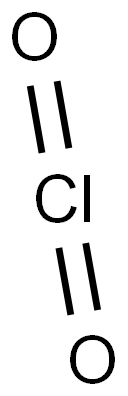
ДИОКСИД ХЛОРА
- английское имяChlorine dioxide
- CAS №10049-04-4
- CBNumberCB5268845
- ФормулаClO2
- мольный вес67.45
- EINECS233-162-8
- номер MDLMFCD01732063
- файл Mol10049-04-4.mol
| Температура плавления | -59°C |
| Температура кипения | 11°C |
| плотность | 3.09g/L |
| давление пара | 0-22900Pa at 20-25℃ |
| растворимость | slightly soluble in H2O |
| форма | orange-green gas |
| цвет | orange-green |
| Растворимость в воде | Soluble ºC |
| Пределы воздействия | TLV-TWA 0.1 ppm (0.3 mg/m3); (ACGIH, MSHA, OSHA, and NIOSH); TLV-STEL 0.3 ppm (ACGIH); IDLH 10 ppm (NIOSH). |
| Диэлектрическая постоянная | 1.7(77.0℃) |
| Стабильность | May decompose explosively on shock, friction or concussion, or on heating rapidly. Strong oxidant - reacts violently with combustible and reducing materials, and with mercury, ammonia, sulphur and many organic compounds. |
| Стандарт первичной питьевой воды EPA | MCL:MRDL=0.81,MCLG:MRDLG=0.81 |
| LogP | -3.22--2.9 at 20℃ |
| Справочник по базе данных CAS | 10049-04-4(CAS DataBase Reference) |
| FDA 21 CFR | 173.300; 137.105; 165.110 |
| Вещества, добавляемые в пищу (ранее EAFUS) | CHLORINE DIOXIDE |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | 8061YMS4RM |
| Справочник по химии NIST | Chlorine dioxide(10049-04-4) |
| Система регистрации веществ EPA | Chlorine dioxide (10049-04-4) |
| Пестициды Закон о свободе информации (FOIA) | Chlorine dioxide |
| Коды опасности | O,T+,N |
| Заявления о рисках | 6-8-26-34-50 |
| Заявления о безопасности | 23-26-28-36/37/39-38-45-61 |
| РИДАДР | UN 9191 |
| OEB | C |
| OEL | TWA: 0.1 ppm (0.3 mg/m3), STEL: 0.3 ppm (0.9 mg/m3) |
| Банк данных об опасных веществах | 10049-04-4(Hazardous Substances Data) |
| ИДЛА | 5 ppm |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H400:Чрезвычайно токсично для водных организмов.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P310:Немедленно обратиться за медицинской помощью.
P363:Перед повторным использованием выстирать загрязненную одежду.
P391:Ликвидировать просыпания/проливы/утечки.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
ДИОКСИД ХЛОРА химические свойства, назначение, производство
Химические свойства
Chlorine dioxide,CI02, is a yellow-reddish gas.It is a very effective bleaching and water treatment agent. Chlorine dioxide is preparedby the reaction of chlorine and sodium chlorite. It is quite unstable and is commonly prepared immediately before use.Физические свойства
Yellow to red-yellow gas at room temperature; pungent chlorine-like odor; density 9.99 g/L at 11°C; liquefies to a reddish brown liquid at 11°C; liquid density 1.64 g/mL at 0°C; freezes at -59.5° C to red crystals (explodes); soluble in water, decomposes in hot water; soluble in alkalis and H2SO4.Использование
Chlorine Dioxide is a gas used in bleaching and aging flour. it acts on the flour almost instantly, resulting in improved color and dough properties. because usage levels are low, the bleaching action is limited.Подготовка
Chlorine dioxide is prepared by passing nitrogen dioxide through sodium chlorate packed in a column:NaClO3 + NO2 → NaNO3 + ClO2
Also, it may be prepared by the reaction of chlorine with sodium chlorite:
2NaClO2 + Cl2 → 2ClO2 + 2NaCl
Alternatively, it may be obtained by the treatment of sodium chlorate or potassium chlorate with sulfur dioxide and sulfuric acid:
2NaClO3 + SO2 + H2SO4 → 2ClO2 + 2 NaHSO4.
Методы производства
Chlorine dioxide is manufactured from the oxidation of chlorite or the reduction of chlorate. The latter method is used for large-volume production and is carried out in strongly acidic solution using reducing agents such as NaCl, HCl, sulfur dioxide, and methanol.Определение
An orange gas formed by the action of concentrated sulfuric acid on potassium chlorate. It is a powerful oxidizing agent and its explosive properties in the presence of a reducing agent were used to make one of the first matches. It is widely used in the purification of water and as a bleach in the flour and wood-pulp industry. On an industrial scale an aqueous solution of chlorine dioxide is made by passing nitrogen dioxide up a tower packed with a fused mixture of aluminum oxide and clay, down which a solution of sodium chlorate flows.Опасность
Explodes when heated or by reaction with organic materials. Very irritating to skin and mucous membranes. Lower respiratory tract irritant. Broncitis.Угроза здоровью
Chlorine dioxide is highly irritating to theeyes, nose, and throat. Inhalation can causecoughing, wheezing, respiratory distress, andcongestion in the lungs. Its toxicity inhumans is moderate to high. Its irritanteffects in humans can be intense at a con centration level of 5 ppm in air. A concen tration of 19 ppm of the gas inside a bleachtank caused the death of one worker (Elkins 1959). The chronic toxicity signs are mainlydyspnea and asthmatic bronchitis, and in cer tain cases irritation of the gastrointestinaltract. Ingestion of the liquid may cause som nolence and respiratory stimulation.Пожароопасность
Nonflammable gas; however, it is highly reactive and a strong oxidizing agent. Chlo rine dioxide explodes violently upon heating, exposure to sunlight, contact with dust, or when subjected to a spark. Detonation occurs at concentrations above 10% in air in the presence of an energy source or catalyst. It undergoes violent reactions with organic matter; explosion occurs when the mixture is subjected to shock or a spark. It reacts spon taneously with sulfur or phosphorus, caus ing ignition and/or explosion. Liquid chlorine dioxide may explode violently when mixed with mercury, caustic potash, caustic soda, or many metal hydrides. The gas reacts explo sively with fluorine and with difluoroamine (Lawless and Smith 1968).Профиль безопасности
Moderately toxic by inhalation. Experimental reproductive effects. Mutation data reported. An eye irritant. A powerful explosive sensitive to spark, impact, sunlight, or heating rapidly to 100℃. A powerful oxidzer. Concentrations of greater than 10% in air are explosive. Explodes on mixing with carbon monoxide, hydrocarbons (e.g., butadiene, ethane, ethylene, methane, propane), fluoramines (e.g., difluoramine, trifluoramine). Mtxtures with hydrogen explode with sparking or contact with platinum. Explodes on contact with mercury, potassium hydroxide, phosphorus pentachloride + chlorine. Ignites or explodes on contact with nonmetals (e.g., phosphorus, sulfur, sugar). Reacts violently with F2, NHF2. Reacts with water or steam to produce toxic and corrosive fumes of HCl. When heated to decomposition it emits toxic fumes of Cl-. See also CHLORINE.Возможный контакт
Chlorine dioxide is used in bleaching cellulose pulp; bleaching flour; water purification; as a liquid sterilizer in an ultrasonic cleaner.Перевозки
UN/NA 9191 Chlorine dioxide, hydrate, frozen, Hazard class: 5.1; Labels: 5.1-Oxidizer, 6.1-Poison Inhalation. Explosive: It may only be shipped in the frozen state and then only by private or contract motor carrier.Несовместимости
Unstable in light. A powerful oxidizer. Chlorine dioxide gas is explosive at concentrations over 10% and can be ignited by almost any form of energy, including sunlight, heat (explosions can occur in air in temperature above 130C), or sparks, shock, friction, or concussion. This chemical reacts violently with dust, combustible materials; and reducing agents. Reacts violently with mercury, phosphorus, sulfur, and many compounds, causing fire and explosion hazard. Contact with water forms perchloric and hydrochloric acid. Corrosive to metals.Утилизация отходов
Use large volume of concentrated solution of ferrous salt or bisulfite solution as reducing agent. Then neutralize and flush to sewer with abundant water.ДИОКСИД ХЛОРА запасные части и сырье
ДИОКСИД ХЛОРА поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +8617531153977 | China | 5855 | 58 | |
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-13734021967 +8613734021967 |
China | 1001 | 58 | |
| +86-86-5926051114 +8618959220845 |
China | 6383 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 |