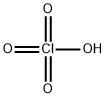
Хлорная кислота
- английское имяPERCHLORIC ACID
- CAS №7601-90-3
- CBNumberCB4348487
- ФормулаClHO4
- мольный вес100.46
- EINECS231-512-4
- номер MDLMFCD00011325
- файл Mol7601-90-3.mol
| Температура плавления | -18 °C |
| Температура кипения | 203 °C |
| плотность | 1.664 g/mL at 25 °C |
| плотность пара | ~2.1 (vs air) |
| давление пара | 6.8 mm Hg ( 25 °C) |
| показатель преломления | 1.419 |
| Fp | 104 °F |
| температура хранения | Flammables area |
| растворимость | Water (Sparingly) |
| пка | -7[at 20 ℃] |
| форма | Solution |
| цвет | APHA: ≤10 |
| Удельный вес | approximate 1.54 |
| Запах | Odorless |
| РН | 0.1 (H2O, 20°C) |
| Растворимость в воде | Miscible with water. |
| Мерк | 14,7153 |
| Стабильность | Stable. Avoid heat. May form explosive peroxides. Incompatible with a wide variety of substances, including organic materials, alcohols, amines, strong acids, strong bases, acid anhydrides, finely powdered metals, strong reducing agents. Contact with wood, paper and other celullose products may lead to explosion, as may contact with a vari |
| ИнЧИКей | VLTRZXGMWDSKGL-UHFFFAOYSA-N |
| LogP | -4.62 |
| Справочник по базе данных CAS | 7601-90-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | V561V90BG2 |
| Справочник по химии NIST | Perchloric acid(7601-90-3) |
| Система регистрации веществ EPA | Perchloric acid (7601-90-3) |
| UNSPSC Code | 12352302 |
| NACRES | NB.21 |
| Коды опасности | C,O,Xi | |||||||||
| Заявления о рисках | 5-8-35-10-34-36/38 | |||||||||
| Заявления о безопасности | 23-26-36-45-36/37/39 | |||||||||
| РИДАДР | UN 2920 8/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | SC7500000 | |||||||||
| F | 3 | |||||||||
| Температура самовоспламенения | 485 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 3822 00 00 | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 7601-90-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 oral (rat) 1100 mg/kg LD50 oral (dog) 400 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
H271:Сильный окислитель; может вызвать возгорание или взрыв.
H290:Может вызывать коррозию металлов.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P314:В случае плохого самочувствия обратиться к врачу.
Хлорная кислота химические свойства, назначение, производство
Химические свойства
Perchloric acid, HCIO4, also known as Fraude's reagent,is a colorless, fuming,hygroscopic liquid that boils at 16°C(61OF). It is a strong oxidizer and is soluble in water. Cold dilute perchloric acid reacts with metals such as zinc and iron to yield hydrogen gas and the metallic perchlorate. Perchloric acid is used in electrolytic baths, electropolishing, explosives, analytical chemistry, and medicine.Физические свойства
Perchloric acid, HClO4, is a colorless liquid soluble in water. It is a strong acid comparable in strength to sulfuric and nitric acids. It is useful for preparing perchlorate salts, but it is also dangerously corrosive and readily forms explosive mixtures. Perchloric acid is produced by the treatment of sodium perchlorate with sulfuric acid and by the electrochemical oxidation of aqueous chlorine.Использование
Perchloric acid salts are used as explosivesand in metal plating. They are also used as anoxidizer and as a reagent in chemical analysis. These salts are produced by distillingpotassium chlorate with concentrated H2SO4under reduced pressure..Общее описание
A clear colorless odorless aqueous solution. Corrosive to metals and tissue. Closed containers may rupture violently under prolonged exposure to heat.Реакции воздуха и воды
Water soluble with heat generation.Профиль реактивности
PERCHLORIC ACID is a solution of a strong oxidizing acid. May react vigorously or deflagrate when mixed with oxidizable material [Merck]. This includes (but is not limited to) alcohols, amines, boranes, dicyanogen, hydrazines, hydrocarbons, hydrogen, nitroalkanes, powdered metals, silanes, or thiols [Bretherick 1979. p.174]. Perchloric acid ignites on contact with sulfinyl chloride. (Bailar, 1973, Vol. 2, 1442).Угроза здоровью
Perchloric acid is a highly corrosive substance that causes severe burns on contact with the eyes, skin, and mucous membranes. The acute toxicity of perchloric acid is moderate. This substance is a severe irritant to the eyes, mucous membranes, and upper respiratory tract. Perchlorates are irritants to the body wherever they contact it. Perchloric acid has not been shown to be carcinogenic or to show reproductive or developmental toxicity in humansПожароопасность
Perchloric acid is noncombustible. The anhydrous (dehydrated) acid presents a serious explosion hazard. It is unstable and can decompose explosively at ordinary temperatures or in contact with many organic compounds.Many heavy metal perchlorates and organic perchlorate salts are extremely sensitive explosives; the ammonium, alkali metal, and alkali earth perchlorates are somewhat less hazardous. Mixtures of perchlorates with many oxidizable substances are explosive.
Воспламеняемость и взрывоопасность
Perchloric acid is noncombustible. The anhydrous (dehydrated) acid presents a serious explosion hazard. It is unstable and can decompose explosively at ordinary temperatures or in contact with many organic compounds.Many heavy metal perchlorates and organic perchlorate salts are extremely sensitive explosives; the ammonium, alkali metal, and alkali earth perchlorates are somewhat less hazardous. Mixtures of perchlorates with many oxidizable substances are explosive.
Экологическая судьба
Perchloric acid, in the presence of moisture, forms the negatively charged perchlorate anion. The largest natural deposit of perchlorate is located in Chile; the origin of the deposit is not known.Atmospheric perchlorate may be found near the sites where it is manufactured and the locations where it is used. Accidental spills of perchloric acid are another source of airborne perchlorate. Perchlorate has a low vapor pressure and is not found in the atmosphere as such; however, airborne particles are known to be a source of perchlorate. The particles may fall to the soil or be washed to the soil via rain. Soil particles containing perchlorate can migrate in air currents or with surface water or groundwater.
Perchlorate anions are highly mobile in groundwater because of their charged state and because they adsorb to soil particles poorly. Perchlorates in groundwater or surface water are extremely persistent. They are extremely stable under ambient conditions and tend not to react or degrade. Some types of anaerobic bacteria are known to biodegrade perchlorate; however, they are effective only under specific environmental conditions (high levels of organic carbon and low levels of oxygen and nitrate). Groundwater extraction is considered inefficient for the removal of perchlorate.
Plants exposed to perchlorate in the soil moisture can also take up perchlorate; some types of plants are known to concentrate perchlorate.
хранилище
Splash goggles and rubber gloves should be worn when handling perchloric acid, and containers of the acid should be stored in a well-ventilated location separated from organic substances and other combustible materials. Work with >85% perchloric acid requires special precautions and should be carried out only by specially trained personnel.Методы очистки
The 72% acid is been purified by double distillation from silver oxide under vacuum: this frees the acid from metal contamination. Distillation at atmospheric pressure is dangerous and explosive. The anhydrous acid is obtained by adding gradually 400-500mL of oleum (20% fuming H2SO4) to 100-120mL of 72% HClO4 in a reaction flask cooled in an ice-bath. The pressure is reduced to 1mm (or less), with the reaction mixture at 20-25o. The temperature is gradually raised during 2hours to 85o; the distillate is collected in a receiver cooled in Dry-ice. For further details of the distillation apparatus see Smith [J Am Chem Soc 75 184 1953]. It is HIGHLY EXPLOSIVE; a strong protective screen should be used at all times. [Schmeisser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 318-320 1963.]Несовместимости
Cold 70% perchloric acid is a strong acid but is not considered to be a strong oxidizing agent; however, more concentrated solutions are good oxidizers. Temperature increases the oxidizing power of perchloric acid, and hot concentrated solutions are very dangerous. Evaporation of a spill of the 70% solution may lead to the formation of more dangerous concentrations. Reaction of 70% perchloric acid with cellulose materials such as wood, paper, and cotton can produce fires and explosions. Oxidizable organic compounds including alcohols, ketones, aldehydes, ethers, and dialkyl sulfoxides can react violently with concentrated perchloric acid. All perchlorates are potentially hazardous when in contact with reducing agents.Утилизация отходов
Excess perchloric acid and waste material containing this substance should be placed in an appropriate container, clearly labeled, and handled according to your institution's waste disposal guidelines.Хлорная кислота запасные части и сырье
сырьё
1of2
запасной предмет
- Jasmone
- 5-AMINO-2-FLUOROPYRIMIDINE
- (R)-3-tert-Butyloxycarbonyl-2-methylpropanoic acid
- (+)-DISPARLURE
- POLYTHIOPHENE
- 3-nitro-4-piperazin-1-ylbenzonitrile
- 4-Бром-3-нитробензонитрила
- трет-Бутилкарбамат
- АЛЬФА-D-ГЛЮКОПИРАНОЗА 1-ФОСФАТ ДИПОТАССИЕВОЙ СОЛИ ГИДРАТ
- O-Mesitylenesulfonylhydroxylamine
1of4