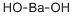
Гидроксид бария
- английское имяBarium hydroxide
- CAS №17194-00-2
- CBNumberCB5118700
- ФормулаBaH2O2
- мольный вес171.34
- EINECS241-234-5
- номер MDLMFCD00003443
- файл Mol17194-00-2.mol
| Температура плавления | >300 °C(lit.) |
| Температура кипения | decomposes at 1032 (calculated) [KNA91] |
| плотность | 2.2 g/mL at 25 °C(lit.) |
| растворимость | Aqueous Acid (Slightly), Water (Slightly) |
| форма | Solid |
| цвет | Clear |
| РН | 11.27(1 mM solution);12.22(10 mM solution);13.08(100 mM solution) |
| Запах | no odor |
| Растворимость в воде | Soluble in water. |
| Чувствительный | Air Sensitive & Hygroscopic |
| Мерк | 14,977 |
| Пределы воздействия | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| Стабильность | Stable. Incompatible with acids, carbon dioxide, moisture. |
| ИнЧИКей | RQPZNWPYLFFXCP-UHFFFAOYSA-L |
| Справочник по базе данных CAS | 17194-00-2(CAS DataBase Reference) |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | BARIUM HYDROXIDE |
| FDA 21 CFR | 177.2410 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 1OHB71MYBK |
| Справочник по химии NIST | Barium dihydroxide(17194-00-2) |
| Система регистрации веществ EPA | Barium hydroxide (Ba(OH)2) (17194-00-2) |
| UNSPSC Code | 12352303 |
| NACRES | NA.25 |
| Коды опасности | C,Xi,Xn | |||||||||
| Заявления о рисках | 36/37/38-34-20/22-36/38-41-35-22 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-36/37 | |||||||||
| РИДАДР | UN 3262 8/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | CQ9200000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 2805191000 | |||||||||
| Банк данных об опасных веществах | 17194-00-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 ipr-mus: 255 mg/kg GTPZAB 28(6),45,84 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302+H332:Вредно при проглатывании или при вдыхании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Гидроксид бария химические свойства, назначение, производство
Описание
Barium Hydroxide occurs as an anhydrate whose prismatic, colorless crystals are very deliquescent. Barium hydroxide can be prepared by dissolving barium oxide (BaO) in water:BaO+ 9H2O→Ba(OH)2·8H2O
It crystallizes as the octahydrate, which converts to the monohydrate upon heating in air. Barium oxide has been found to react with water vapor at temperatures from 1443 to 1593 K to form gaseous Ba(OH)2 according to the reaction:
BaO(solid)+H2O(gas)→Ba(OH)2(gas)
A better method of preparation involves addition of NaOH to the nitrate. Since barium hydroxide is fairly soluble, it is necessary to evaporate the solution to crystallize Ba(OH)2·8H2O. Ethanol is added to aid in this crystallization. The crystals are washed in cold ethanol to remove sodium ions and then dried.
Химические свойства
Barium hydroxide,Ba(OH)2·8H20, is a white crystalline powder, colorless solid composed of monoclinic crystals. It has a melting point of 78°C and is soluble in water and insoluble in acetone. It is used in the fusion of silicates and fat saponification.Физические свойства
Monohydrate, Ba(OH)2?H2O is a white powder; density 3.743 g/cm3; slightly soluble in water; soluble in dilute mineral acids. Octahydrate, Ba(OH)2?8H2O is a colorless monoclinic crystal; density 2.18 g/cm3 at 16°C; refractive index 1.50; melts at 78°C; vapor pressure 227 torr; loses seven molecules of water of crystallization when its solution is boiled in the absence of atmospheric CO2 forming solid monohydrate; further heating produces anhydrous Ba(OH)2 melting at 407°C; readily dissolves in water (3.76 g/100 g at 20°C and 11.7 g/100 g at 50°C); aqueous solution highly alkaline; also soluble in methanol; slightly soluble in ethanol; insoluble in acetone.Использование
Barium hydroxide is used in analytical chemistry to titrate weak acids, particularly organic acids. It forms clear aqueous solutions that are free from carbonate, unlike those of the alkali hydroxides, since barium carbonate is insoluble in water. This allows the use of indicators such as phenolphthalein or thymophthalein (with alkaline color changes) without the risk of titration errors due to the presence of weakly basic CO32- ions.Barium hydroxide is used in organic synthesis as a strong base, for example for the hydrolysis of esters and nitriles.It has been used to hydrolyze one of the two equivalent ester groups in dimethyl endecanedioate. It is also used in the preparation of cyclopentanone, diacetone alcohol and d-Gluonic g-lactone.Barium hydroxide is used as an additive in thermoplastics (such as phenolic resins), rayon and PVC stabilizers to improve plastic properties. This material is used as a general-purpose additive for lubricants and greases. Other industrial applications for barium hydroxide include sugar fabrication, manufacturing soaps, fat saponification, fusing of silicates and chemical synthesis of other barium compounds and organic compounds.
Barium hydroxide is offered for sale both as the octahydrate and the monohydrate. It is available in large quantities commercially.
Подготовка
Barium hydroxide is made by dissolving barium oxide in hot water. The octahydrate, Ba(OH)2?8H2O, crystallizes upon cooling. It also is prepared by precipitation with caustic soda from an aqueous solution of barium sulfide:BaS + 2NaOH → Ba(OH)2 + Na2S.
Опасность
An acute poison; toxic symptoms are similar to other soluble salts of barium (see Barium).Профиль безопасности
A poison by ingestion andintraperitoneal routes. Incompatible with chlorinated rubber.Гидроксид бария запасные части и сырье
Гидроксид бария поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-024-23769576 +86-15040101888 |
China | 254 | 58 | |
| +8618523575427 | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +8617531153977 | China | 5855 | 58 |