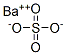
Барий сернокислый
- английское имяBarium sulfate
- CAS №7727-43-7
- CBNumberCB9854295
- ФормулаBaO4S
- мольный вес233.39
- EINECS231-784-4
- номер MDLMFCD00003455
- файл Mol7727-43-7.mol
| Температура плавления | 1580 °C | ||||||||||||||
| Температура кипения | decomposes at 1580℃ [KIR78] | ||||||||||||||
| плотность | 4.5 | ||||||||||||||
| Плотность накопления | 500kg/m3 | ||||||||||||||
| температура хранения | Storage temperature: no restrictions. | ||||||||||||||
| растворимость | water: insoluble | ||||||||||||||
| форма | powder | ||||||||||||||
| Удельный вес | 4.5 | ||||||||||||||
| цвет | White to yellow | ||||||||||||||
| РН | 3.5-10.0 (100g/l, H2O, 20℃)suspension | ||||||||||||||
| Водородный показатель | 7 | ||||||||||||||
| Запах | wh. or ylsh. fine powd. free from grittiness, odorless, tasteless | ||||||||||||||
| Растворимость в воде | 0.0022 g/L (50 ºC) | ||||||||||||||
| Кристальная структура | Barite | ||||||||||||||
| crystal system | Nogata | ||||||||||||||
| Мерк | 14,994 | ||||||||||||||
| Константа произведения растворимости (Ksp) | pKsp: 9.97 | ||||||||||||||
| Space group | Pnma | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Диэлектрическая постоянная | 11.4(16.0℃) | ||||||||||||||
| Пределы воздействия | ACGIH: TWA 5 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: TWA 10 mg/m3; TWA 5 mg/m3 |
||||||||||||||
| Стабильность | Stable. | ||||||||||||||
| LogP | -1.031 (est) | ||||||||||||||
| Справочник по базе данных CAS | 7727-43-7(CAS DataBase Reference) | ||||||||||||||
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | BARIUM SULFATE | ||||||||||||||
| FDA 21 CFR | 175.105; 176.170; 177.2600; 178.3297 | ||||||||||||||
| Рейтинг продуктов питания EWG | 2 | ||||||||||||||
| Словарь онкологических терминов NCI | barium sulfate; barium swallow | ||||||||||||||
| FDA UNII | 25BB7EKE2E | ||||||||||||||
| Система регистрации веществ EPA | Barium sulfate (7727-43-7) | ||||||||||||||
| UNSPSC Code | 12352302 | ||||||||||||||
| NACRES | NA.55 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 20/21/22-36/37/38 | |||||||||
| Заявления о безопасности | 22-24/25-36-26 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 10 mg/m3 (total) | |||||||||
| WGK Германия | - | |||||||||
| RTECS | CR0600000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28332700 | |||||||||
| Банк данных об опасных веществах | 7727-43-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: > 20000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P332+P313:При возникновении раздражения кожи: обратиться за медицинской помощью.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
P362+P364:Снять всю загрязненную одежду и выстирать ее перед повторным использованием.
Барий сернокислый химические свойства, назначение, производство
Описание
Barium sulfate is available as odourless, tasteless, white or yellowish crystals or powder or polymorphous crystals. It is stable and insoluble or negligibly soluble in water, and on burning, it may produce sulphur oxides. It reacts violently with aluminium powder. It occurs naturally as mineral barite, barytes. It has wide use as inert filler and pigment extender in paints, primers, inks, plastics, floor tiles, paper coatings, polymer fibres, and rubber. It is used as the semi-transparent base (lake) for organic pigments and as a thixotropic weighting mud in oil well drilling. Barium sulphate is a contrast agent that is used to help x-ray diagnosis of problems in areas of the upper GI tract, like the oesophagus, the stomach, and/ or the small intestine. The raw barium sulphate has wide applications such as in the manufacture of lithopone, a white pigment in the manufacture of photographic paper, wallpaper and glassmaking, in battery plate expanders, and in heavy concrete for radiation shield.Химические свойства
Barium sulfate is available as white powder or polymorphous crystals. It is stable, odor- less, insoluble, or negligibly soluble in water, and may produce sulfur oxides on burning. It occurs naturally as mineral barite (barytes). It has wide use as an inert i ller pigment extender in paints, primers, inks, plastics, l oor tiles, paper coatings, polymer i bers, and rubber. It is used as the semi-transparent base (lake) for organic pigments and as a thixo- tropic weighting mud in oil well drilling. Barium sulfate is radio-opaque and is used as bar- ium meal in medical x-ray diagnosis. Barium sulfate is a contrast agent that is used to help x-ray diagnosis of problems in areas of the upper GI tract, like the esophagus, the stomach, and/or the small intestine. Barium sulfate is the raw material for the manufacture of litho- pone, a white pigment, and is used in the manufacture of photographic paper, wallpaper, and glassmaking; in battery plate expanders; and in heavy concrete for radiation shield.Вхождение
Barium sulfate is widely distributed in nature and occurs as the mineral barite (also known as barytes or heavy spar). It often is associated with other metallic ores, such as fluorspar. Barites containing over 94% BaSO4 can be processed economically.Barium sulfate has many commercial applications. It is used as natural barite, or precipitated BaSO4. The precipitated salt in combination with equimolar amount of co-precipitated zinc sulfide formerly was used as a white protective coating pigment, known as lithophone. Similarly, in combination with sodium sulfide, it is used to produce fine pigment particles of uniform size, known as blanc fixe. Natural barite, however, has greater commercial application than the precipitated salt. It is used as drilling mud in oil drilling to lubricate and cool the drilling bit, and to plaster the walls of the drill hole to prevent caving. It is used as a filler in automotive paints, plastics and rubber products. It also is used in polyurethane foam floor mats; white sidewall rubber tires; and as a flux and additive to glass to increase the refractive index.
Other chemical applications of barium sulfate are as the opaque ingredient in a barium meal for x-ray diagnosis; as a pigment for photographic paper; and to prepare many barium salts.
Использование
barium sulfate is an emulsion stabilizer for sunscreen formulations. outside of sunscreen preparations, this inorganic salt is most commonly used in non-cosmetic soaps.Определение
ChEBI: A metal sulfate with formula BaO4S. Virtually insoluble in water at room temperature, it is mostly used as a component in oil well drilling fluid it occurs naturally as the mineral barite.Методы производства
Natural barium sulfate or barite is widely distributed in nature. It also contains silica, ferric oxide and fluoride impurities. Silica is the prime impurity which may be removed as sodium fluorosilicate by treatment with hydrofluoric acid followed by caustic soda.Very pure barium sulfate may be precipitated by treating an aqueous solution of a barium salt with sodium sulfate:
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Precipitated BaSO4 is often used in many industrial applications. Blanc fixe and Lithopone are made by the reactions of barium sulfide with sodium sulfate and zinc sulfate, respectively.
Общее описание
Barium sulphate is widely employed as an inorganic filler. Its crystals belong to the rhombic crystal system. The rhombic crystals transforms to monoclinic form at 1148°C and eventually it decomposes to barium oxide, sulfur dioxide and oxygen at temperatures above 1400°C.Профиль реактивности
Barium sulfate is non-combustible and non-toxic. Emits toxic sulfur oxides when heated to decomposition. Can act as an oxidizing agent, but usually does not. Reacts with reducing agents such as potassium, phosphorus or aluminum (heating with aluminum can cause an explosion).Опасность
Pneumoconiosis.Угроза здоровью
Exposures to barium sulfate cause irritation to the eyes, lachrimation; redness, scaling, and itching are characteristics of skin inl ammation. Although barium sulfate has been identi- i ed as a non-toxic dust, long-term inhalation of dust in high concentrations has caused benign pneumoconiosis (baritosis), deposition of dust in the lungs in sufi cient quantities to produce adverse effects. This produces a radiological picture even though symptoms and abnormal signs may not be present. The Fumes of barium are respiratory irritants and over-exposure to dusts and fumes is known to cause rhinitis, frontal headache, wheezing, laryngeal spasm, salivation, and anorexia. Long-term effects include nervous disorders and adverse effects on the heart, circulatory system, and musculature. Heavy exposures to barium sulfate may cause benign pneumoconi in exposed workers. However, there are no reports indicating that barium sulfate has potential occupational hazards or carcinogenicity.Фармацевтические приложения
Barium salts can be highly toxic even at low concentrations. Barium carbonate is highly toxic and can be used as rat poison as it readily dissolves in the stomach acid. Barium sulfate is the least toxic barium compound mainly because of its insolubility. Barium sulfate is used in a variety of applications ranging from white paint to X-ray contrast agent. The clinical use of barium sulfate suspension is well known under the term barium meal. Patients are given a suspension of barium sulfate to swallow. Using X-ray imaging, the whole oesophagus, the stomach and the intestines can be visualised. Barium sulfate lines the tissue whilst travelling through the digestive tract. The heavy barium ions absorb X-rays readily and therefore these structures become visible in an X-ray screening. Barium sulfate is a well-used and tolerated oral radio-contrast agent. It is also used as radio-contrast agent in enemas.Профиль безопасности
Questionable carcinogen with experimental tumorigenic data. Mutation data reported. A relatively insoluble salt used as an opaque medium in radtography. Soluble impurities can lead to toxic reactions. Heating with aluminum can produce an explosion. Incompatible with aluminum and potassium. When heated to decomposition it emits toxic fumes of SOx.Возможный контакт
Barium sulfate is used as an opaque medium in radiography; as a mud weighting material in oil well drilling; in paper coating; as a paint pigment.хранилище
Barium sulfate should be kept stored in its original sealed glass container with proper security when not in use. Barium sulfate should be stored in a cool, dry, ventilated area, away from incompatible materials and foodstuff containers. The containers need to be protected against physical damage and checked regularly for leaks.Перевозки
UN1564 Barium compounds, n.o.s., Hazard Class: 6.1; Labels: 6.1—Poisonous materials.Несовместимости
May act as an oxidizer. Reacts with reducing agents such as hydrides, potassium, phosphorus or aluminum. Aluminum powder1heat may be violent; possibly explosive.Меры предосторожности
Barium sulfate should only be used by or under the direct supervision of a qualii ed super- visor or doctor. During use and handling of barium sulfate, workers should avoid contact of the chemical substance with the skin and should not breathe dust. After use, the work- ers should wash their hands with soap and water. While dispensing, care is needed to label barium sulfate properly and correctly, to avoid confusion with poisonous barium suli de, suli te, or carbonate.Барий сернокислый запасные части и сырье
сырьё
1of3
запасной предмет
- Various color alkyd ready mixed paint
- Alkyd resin paint
- Бария октагидрат гидроксид
- Натрий сульфид нонагидрат
- Бария нитра
- Various color alkyd primer
- Barium sulfate,superfine
- Бария карбонат
- 1-гексен
- Red lead alkyd antirust paint
- Бария хлорида дигидра
- Натрия сульфид
- Barium naphthenate
- Моногидрат гидроксида бария
- MANGANESE(II) HYPOPHOSPHITE MONOHYDRATE
1of6
Барий сернокислый поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-18034019111 | China | 255 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| 18871490254 | CHINA | 28172 | 58 |