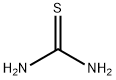
Тиомочевина
- английское имяThiourea
- CAS №62-56-6
- CBNumberCB9854008
- ФормулаCH4N2S
- мольный вес76.12
- EINECS200-543-5
- номер MDLMFCD00008067
- файл Mol62-56-6.mol
| Температура плавления | 170-176 °C (lit.) |
| Температура кипения | 263.89°C (estimate) |
| плотность | 1.405 |
| Плотность накопления | 640kg/m3 |
| показатель преломления | 1.5300 (estimate) |
| температура хранения | Store below +30°C. |
| растворимость | water: soluble137g/L at 20°C |
| форма | Crystals |
| пка | -1.0(at 25℃) |
| Удельный вес | 1.406 |
| цвет | White to almost white |
| РН | 6-8 (50g/l, H2O, 20℃) |
| Запах | Odorless |
| Водородный показатель | 5 - 7 |
| Растворимость в воде | 13.6 g/100 mL (20 ºC) |
| Мерк | 14,9367 |
| БРН | 605327 |
| Стабильность | Stable. Incompatible with strong acids, strong bases, strong oxidizing agents, metallic salts, proteins, hydrocarbons. May react violently with acrolein. |
| ИнЧИКей | UMGDCJDMYOKAJW-UHFFFAOYSA-N |
| LogP | -1.050 (est) |
| FDA 21 CFR | 189.190 |
| Вещества, добавляемые в пищу (ранее EAFUS) | THIOUREA--PROHIBITED |
| Справочник по базе данных CAS | 62-56-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 5-8 |
| Словарь онкологических терминов NCI | THU |
| FDA UNII | GYV9AM2QAG |
| Предложение 65 Список | Thiourea |
| Справочник по химии NIST | Thiourea(62-56-6) |
| МАИР | 3 (Vol. Sup 7, 79) 2001 |
| Система регистрации веществ EPA | Thiourea (62-56-6) |
| UNSPSC Code | 41116107 |
| NACRES | NB.21 |
| Коды опасности | Xn,N,Xi | |||||||||
| Заявления о рисках | 22-40-51/53-63-43-38 | |||||||||
| Заявления о безопасности | 36/37-61 | |||||||||
| РИДАДР | UN 2811 6.1/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | YU2800000 | |||||||||
| Температура самовоспламенения | 440 °C Dust | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29309070 | |||||||||
| Банк данных об опасных веществах | 62-56-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in wild Norway rats: 1830 mg/kg (Dieke) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H351:Предполагается, что данное вещество вызывает раковые заболевания.
H411:Токсично для водных организмов с долгосрочными последствиями.
H361fd:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению. Предполагается, что данное вещество может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P264:После работы тщательно вымыть кожу.
P273:Избегать попадания в окружающую среду.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Тиомочевина химические свойства, назначение, производство
Описание
Thiourea appears as white crystal/powder, is combustible, and on contact with fire, gives off irritating or toxic fumes/gases. Thiourea is a reducing agent used primarily in the production of bleached recycled pulp. In addition, it is also effective in the bleaching of stone groundwood, pressurised groundwood. Thiourea undergoes decomposition on heating and produces toxic fumes of nitrogen oxides and sulphur oxides. It reacts violently with acrolein, strong acids, and strong oxidants. The main application of thiourea is in textile processing and also is commonly employed as a source of sulphide. Thiourea is a precursor to sulphide to produce metal sulphides, for example, mercury sulphide, upon reaction with the metal salt in aqueous solution. The industrial uses of thiourea include production of flame-retardant resins and vulcanisation accelerators. Thiourea is used as an auxiliary agent in diazo paper, light-sensitive photocopy paper, and almost all other types of copy paper. Thiourea is used in many industrial applications, including as a chemical intermediate or catalyst, in metal processing and plating, and in photoprocessing.Химические свойства
Thiourea consists of colorless, lustrous crystals or powder with a bitter taste.Использование
The product is wildly used in pharmaceutical industry, agricultural, chemicals, metallurgical industry, petroleum and so on. It is also main material for producing thiourea dioxide(CH1N2O2S).Подготовка
Thiourea is manufactured by heating ammonium thiocyanate at 140-145??C for about 4 hours; equilibrium is established when about 25% of the thiocyanate is converted to thiourea.Thiourea may also be prepared by the interaction of cyanamide and hydrogen sulphide: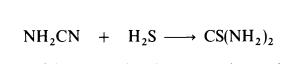
Thiourea closely resembles urea in that reaction with formaldehyde gives methylol derivatives and then resinous condensates which on continued heating yield network structures. Thiourea-formaldehyde resins are slower curing than urea-formaldehyde resins and the hardened products are more brittle and more water-resistant. At one time thiourea-formaldehyde resins were added to urea-formaldehyde resins to give mouldings and laminates with improved water-resistance. These mixed resins have now been largely superseded by melamine-formaldehyde resins which give products with better resistance to heat.
Определение
ChEBI: The simplest member of the thiourea class, consisting of urea with the oxygen atom substituted by sulfur.Методы производства
Thiourea is formed by heating ammonium thiocyanate at 170 °C (338 °F). After about an hour, 25% conversion is achieved. With HCl, thiourea forms thiourea hydrochloride; with mercuric oxide, thiourea forms a salt; and with silver chloride, it forms a complex salt.Общее описание
White or off-white crystals or powder. Sinks and mixes with water.Реакции воздуха и воды
Water soluble.Профиль реактивности
Thiocarbamide is a white crystalline material or powder, toxic, carcinogenic. When heated to decomposition Thiocarbamide emits very toxic fumes of oxides of sulfur and oxides of nitrogen. Violent exothermic polymerization reaction with acrylaldehyde (acrolein) [MCA SD-85, 1961], violent decomposition of the reaction product with hydrogen peroxide and nitric acid [Bjorklund G. H. et al., Trans. R. Soc. Can.,1950, 44, p. 28], spontaneous explosion upon grinding with potassium chlorate [Soothill, D., Safety Management, 1992, 8(6), p. 11].Опасность
A questionable carcinogen. May not be used in food products (FDA); skin irritant (allergenic).Угроза здоровью
The acute oral toxicity of thiourea in mostanimals is of low order. The oral LD50 values reported in the literature show variation.Symptoms of chronic effects in rats includebone marrow depression and goiters. Administration of 32.8 mol of thiourea in chickembryos on day 17 of incubation resultedin the accumulation of parabronchial liquidin those embryos (Wittman et al. 1987). Theinvestigators have attributed such changes tothe toxic effects of thiourea, rather to than aretardation of pulmonary development.Dedon and coworkers (1986) observed thepossible protective action of thiourea againstplatinum toxicity. Thiourea and other sulfur-containing nucleophiles have the ability tochelate and remove platinum from biochemical sites of toxicity.Oral administration of thiourea resultedin tumors in the liver and thyroid in rats.It is carcinogenic to animals and has shownsufficient evidence.Пожароопасность
Noncombustible solid. There is no report of any explosion resulting from reactions of thiourea. Small amounts of thiourea in contact with acrolein may polymerize acrolein, which is a highly exothermic reaction.Сельскохозяйственное использование
Thiourea is a sulphur analogue of urea. It is a crystalline and colorless solid which is relatively insoluble in water. Thiourea, capable of breaking the dormancy of seeds, is used to stimulate seed germination. Seeds are soaked for less than 24 hours before planting.Контактные аллергены
Thiourea is used as a cleaner agent for silver and cop- per, and as an antioxidant in diazo copy paper. It can induce (photo-) contact dermatitis.Возможный контакт
Thiourea is used as rubber antiozonant, toning agent; corrosion inhibitor; and in pharmaceutical manufacture; in the manufacture of photosensitive papers; flame-retardant textile sizes; boiler water treatment. It is also used in photography; pesticide manufacture; in textile chemicals.Канцерогенность
Thiourea is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in experimental animals.Перевозки
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required. UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Методы очистки
Crystallise thiourea from absolute EtOH, MeOH, acetonitrile or water. Dry it under vacuum over H2SO4 at room temperature. [Beilstein 3 IV 342.]Несовместимости
Dust may form explosive mixture with air. Reacts violently with acrolein, strong acids (nitric acid). Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.Утилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.Тиомочевина запасные части и сырье
сырьё
1of2
запасной предмет
- 2-ХЛОР-5-НИТРОТИАЗОЛ
- 4-(4-хлорфенил)пиримидин-2-тиол
- АЦЕТИЛТИОМОЧЕВИНА
- 2-AMINO-6-METHYL-PYRIMIDINE-4-THIOL
- 2-Амино-((1-карбокси-1-метил этокси)имино)-4-тиазолуксусная кислота
- 6-AMINO-2-METHYLTHIO-3-METHYLURACIL
- 4,6-Dimethyl-2-methylmercapyrimidine
- 5-ацетил-2-амино-4-метилтиазол
- Этил 2-хлор-4-метил-1,3-тиазол-5-карбоксила
- Ethyl 4-amino-2-(ethylthio)-5-pyrimidinecarboxylate
- 9,10-диметилантрацен
- 4-AMINO-2-MERCAPTOPYRIMIDINE-5-CARBONITRILE
- 4,6-DIMETHYL-2-THIOPYRIMIDINE
- Этил 2-амино-4-фенил-5-тиазолкарбоксила
- Оксфендазол
- АМИКАРТИАЗОЛ
- ETHYL 2-(ETHYLTHIO)-4-HYDROXYPYRIMIDINE-5-CARBOXYLATE
- Pseudothiohydantoin
- Этил 2-амино-4-метилтиазол-5-карбоксилат
- 4,6-дигидрокси-2-меркаптопиримидин
- 2- (2-аминотиазол-4-ил) -2-метоксииминоуксусная кислота
- 2-Амино-5-метилтиазол
- изобутантиол
- Этил 2- (2-аминотиазол-4-ил) -2-гидроксииминоацетат
- METHYL 2-CHLORO-4-THIAZOLECARBOXYLATE
- 2-амино-2-тиазолина гидрохлорид
- 4-гидрокси-6-меркаптопиразолo[3,4-d]пиримидин
- 4,6-DIAMINO-2-MERCAPTOPYRIMIDINE
- Метиленовый dithiocyanate
- 1-октантиол
1of8
Тиомочевина поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | ||
| +8613573296305 | China | 301 | 58 | ||
| +8618660799346 | China | 1009 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-86-0510-85881806 +8613357920996 |
China | 87 | 58 | ||
| +86-2102300 +86-18632882519 |
China | 300 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 |