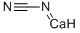
Цианамид кальция
- английское имяCalcium cyanamide
- CAS №156-62-7
- CBNumberCB0297271
- ФормулаCCaN2
- мольный вес80.1
- EINECS205-861-8
- номер MDLMFCD00064894
- файл Mol156-62-7.mol
| Температура плавления | >300 °C(lit.) |
| плотность | 2.29 |
| давление пара | 0.51Pa at 20℃ |
| растворимость | reacts with H2O |
| форма | Powder |
| Удельный вес | 2.29 |
| цвет | Gray to dark gray |
| Запах | Odorless |
| Растворимость в воде | insoluble H2O, but undergoes hydrolysis releasing acetylene and ammonia [HAW93] [MER06] |
| Чувствительный | Moisture Sensitive |
| Мерк | 14,1662 |
| БРН | 4124391 |
| ИнЧИКей | QFSRQFUIHVTIDL-UHFFFAOYSA-N |
| LogP | -0.72 at 20℃ |
| Справочник по базе данных CAS | 156-62-7(CAS DataBase Reference) |
| FDA UNII | ZLR270912W |
| Код УВД | N07BB02 |
| Система регистрации веществ EPA | Calcium cyanamide (156-62-7) |
| Коды опасности | Xn,F | |||||||||
| Заявления о рисках | 22-37-41-15-43-37/38 | |||||||||
| Заявления о безопасности | 26-39-43-36/37/39-22-45-28 | |||||||||
| РИДАДР | UN 1403 4.3/PG 3 | |||||||||
| OEB | C | |||||||||
| OEL | TWA: 0.5 mg/m3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | GS6000000 | |||||||||
| F | 9-21 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 31029010 | |||||||||
| Банк данных об опасных веществах | 156-62-7(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
H318:При попадании в глаза вызывает необратимые последствия.
H317:При контакте с кожей может вызывать аллергическую реакцию.
H261:При контакте с водой выделяет воспламеняющиеся газы.
-
оператор предупредительных мер
P231+P232:Обращаться с продуктом и хранить его в атмосфере инертного газа . Беречь от влаги.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338+P310:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз. Немедленно обратиться за медицинской помощью.
P333+P313:При возникновении раздражения или покраснения кожи обратиться за медицинской помощью.
Цианамид кальция химические свойства, назначение, производство
Химические свойства
Calcium cyanamide is a blackish-gray, shiny crystalline material or powder.
Физические свойства
Pure product is a colorless, hexagonal crystal or white powder. Commercial grade material may be grayish-black powder or lump (the color is due to presence of calcium carbide and other impurities); density 2.29 g/cm3; melts around 1,340°C; sublimes around 1,150 to 1,200°C on rapid heating; reacts with water.Использование
Calcium Cyanamide is used as a fertilizer, herbicide, insecticide, a steel-making additive and an ore processing material. It can also be used to make thiourea, guanidine and ferrocyanides. manufacture of calcium cyanide, melamine, dicyandiamide.Подготовка
Calcium cyanamide is prepared from calcium carbide. The carbide powder is heated at about 1,000°C in an electric furnace into which nitrogen is passed for several hours. The product is cooled to ambient temperatures and any unreacted carbide is leached out cautiously with water.CaC2 + N2 → CaCN2 + C (ΔHƒ°= –69.0 kcal/mol at 25°C)
Методы производства
Calcium cyanamide was first produced commercially around 1900 as a fertilizer. The process of making calcium cyanamide involves three raw materials—coke, coal, and limestone— plus nitrogen. The limestone (calcium carbonate) is burned with coal to produce calcium oxide. The calcium oxide is then allowed to react with amorphous carbon in the furnace at 2000°C with the formation of calcium carbide (CaC2). Finely powdered calcium carbide is heated to 1000°C in an electric furnace into which pure nitrogen is passed. It is then removed and uncombined calcium carbide removed by leaching.Определение
calcium cyanamide: A colourlesssolid, CaCN2, which sublimes at1300°C. It is prepared by heating calciumdicarbide at 800°C in a streamof nitrogen:CaC2(s) + N2(g) → CaCN2(s) + C(s)
The reaction has been used as amethod of fixing nitrogen in countriesin which cheap electricity isavailable to make the calcium dicarbide(the cyanamide process). Calciumcyanamide can be used as afertilizer because it reacts with waterto give ammonia and calcium carbonate:
CaCN2(s) + 3H2O(l) → CaCO3(s) +2NH3(g)
It is also used in the production ofmelamine, urea, and certain cyanidesalts.
Общее описание
A colorless to gray, odorless solid. May cause illness from ingestion. May irritate the skin. If exposed to water or high temperatures, calcium cyanamide may generate toxic and flammable fumes. Used to make pesticides and in fertilizers.Реакции воздуха и воды
Depending on the calcium carbide content, the cyanamide reacts with water (moisture from air or soil) to produce acetylene and hydrated calcium oxide or calcium hydroxide. Absorption of water during handling or storage of technical calcium cyanamide may cause explosion [Pieri, M. Chem. Abs. 46, 8335 1952].Профиль реактивности
When hydrated CALCIUM CARBIDE generates salts of calcium that are basic and are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.Опасность
Fire risk with moisture or combined with calcium carbide. Skin, eye, and upper respiratory tract irritant. Questionable carcinogen.Угроза здоровью
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.Пожароопасность
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.Профиль безопасности
Poison by ingestion, inhalation, sh contact, intravenous, and intraperitoneal routes. Moderately toxic to humans by ingestion. Questionable carcinogen with experimental tumorigenic data. Mutation data reported. The fatal dose, by ingestion, is probably around 20 to 30 g for an adult. It does not have a cyanide effect. Calcium cyanamide is not believed to have a cumulative action. Flammable. Reaction with water forms the explosive acetylene gas. When heated to decomposition it emits toxic fumes of NOx and CN-. See also CALCIUM COMPOUNDS, AMIDES, and CYANIDEВозможный контакт
Calcium cyanamide is used in agriculture as a fertilizer, herbicide; defoliant for cotton plants; and pesticide. It is also used in the manufacture of dicyandiamide and calcium cyanide as a desulfurizer in the iron and steel industry; and in steel hardening.Канцерогенность
Calcium cyanamide was weakly mutagenic in Salmonella typhimurium strain TA1535 and nonmutagenic in strain TA100.Перевозки
UN1403 Calcium cyanamide with .1% calcium carbide, Hazard Class: 4.3; Labels: 4.3-Dangerous when wet materialНесовместимости
Commercial grades of calcium cyanamide may contain calcium carbide; contact with any form of moisture solutions may cause decomposition, liberating explosive acetylene gas and ammonia. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. May polymerize in water or alkaline solutions to dicyanamide. Contact with all solvents tested also causes decompositionЦианамид кальция запасные части и сырье
Цианамид кальция поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +86 18953170293 | China | 2930 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 86-714-3992388 | United States | 14332 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-0571-85134551 | China | 15352 | 58 |