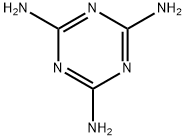
Меламин
- английское имяMelamine
- CAS №108-78-1
- CBNumberCB6324023
- ФормулаC3H6N6
- мольный вес126.12
- EINECS203-615-4
- номер MDLMFCD00131581
- файл Mol108-78-1.mol
| Температура плавления | >300 °C (lit.) |
| Температура кипения | 224.22°C (rough estimate) |
| плотность | 1.573 |
| Плотность накопления | 800kg/m3 |
| давление пара | 66.65 hPa (315 °C) |
| показатель преломления | 1.872 |
| Fp | >110°C |
| температура хранения | no restrictions. |
| растворимость | water: soluble25mg/mL, clear to slightly hazy, colorless |
| пка | 5(at 25℃) |
| форма | Fine Crystalline Powder |
| цвет | White |
| РН | 7-8 (32g/l, H2O, 20℃) |
| Растворимость в воде | 3 g/L (20 ºC) |
| Мерк | 14,5811 |
| БРН | 124341 |
| Стабильность | Stable. Incompatible with strong acids, strong oxidizing agents. Nonflammable. |
| ИнЧИКей | JDSHMPZPIAZGSV-UHFFFAOYSA-N |
| LogP | -1.22 at 20℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | MELAMINE |
| FDA 21 CFR | 175.105 |
| Справочник по базе данных CAS | 108-78-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2 |
| Словарь онкологических терминов NCI | melanin |
| FDA UNII | N3GP2YSD88 |
| МАИР | 2B (Vol. Sup 7, 73, 119) 2019 |
| Справочник по химии NIST | 1,3,5-Triazine-2,4,6-triamine(108-78-1) |
| Система регистрации веществ EPA | Melamine (108-78-1) |
| UNSPSC Code | 85151701 |
| NACRES | NA.24 |
| Коды опасности | Xi,Xn | |||||||||
| Заявления о рисках | 43-44-20/21 | |||||||||
| Заявления о безопасности | 36/37 | |||||||||
| РИДАДР | 3263 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | OS0700000 | |||||||||
| Температура самовоспламенения | >600 °C | |||||||||
| TSCA | Yes | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29336980 | |||||||||
| Банк данных об опасных веществах | 108-78-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 3161 mg/kg LD50 dermal Rabbit > 1000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H361f:Предполагается, что данное вещество может отрицательно повлиять на способность к деторождению.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Меламин химические свойства, назначение, производство
Описание
Melamine-formaldehyde resin (MFR) is an active ingredient of strong (reinforced) plasters. Sensitization was reported in a plaster-room technician, who applied resin-reinforced pIaster casts, and in dental technicians. MFR was contained in a strong dental pIaster used for mouldings. Used as a textile finish res in, it was also found to be an allergen in a women who replaced clothes in a store. MFR also releases formaldehyde, which may be the sensitizer.Химические свойства
Melamine is a white solid organic compound whose molecules consist of a sixmembered heterocyclic ring of alternate carbon and nitrogen atoms with three amino groups attached to the carbons. Condensation polymerization with methanal or other aldehydes produces melamine resins, which are important thermosetting plastics.Использование
It is used to make high-pressure laminating resins (e.g., decorative countertops), molded compounds (e.g., dinnerware), and surface coating resins (e.g., appliance finishes and automotive topcoats). Additional major products are textile and paper treatment resins. Miscellaneous uses include adhesive resins for gluing lumber, plywood, and flooring, and resins for leather tanning agents. Melamine, melamine cyanurate, other melamine salts, and guanidine compounds are currently the most used group of nitrogencontaining flame retardants. Melamine is used as a flame retardant additive for polypropylene and polyethylene. Melamine cyanurate is employed commercially as a flame retardant for polyamides and terephthalates.Методы производства
Melamine is prepared almost exclusively by the urea process—the action of ammonia on urea. It is produced worldwide.Подготовка
The standard route to melamine is from urea. Urea is heated in the presence of ammonia at 250-350??C and 4--20 MPa. The reaction probably involves the simultaneous dehydration and hydration of urea to form cyanamide and ammonium carbamate; trimerization of the cyanamide then leads to melamine: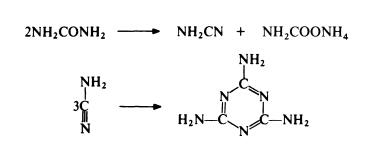
Thus only 50% of the urea used gives melamine in one step and ammonium carbamate has to be separated and converted to urea for recycling. Despite this limitation, the urea route is the most economical of currently available routes.
Общее описание
Colorless to white monoclinic crystals or prisms or white powder. Sublimes when gently heated.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Melamine is incompatible with strong oxidizing agents and strong acids . Neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides.Опасность
Toxic by ingestion, skin, and eye irritant. Questionable carcinogen.Пожароопасность
Literature sources indicate that Melamine is nonflammable.Контактные аллергены
Melamine-formaldehyde resin (MFR) results from condensation of melamine and formaldehyde. It is anactive ingredient of strong (reinforced) plasters, such as industrial or some dental plasters used for molding.It is also used as a textile finish resin. MFR acts as an allergen generally because of formaldehyde releasing (see Chap. 40)Профиль безопасности
Moderately toxic by ingestion and intraperitoneal routes. An eye, skin, and mucous membrane irritant. Causes dermatitis in humans. Questionable carcinogen with experimental carcinogenic and tumorigenic data. Experimental reproductive effects. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx and CN-.Возможный контакт
Manufactured from urea, melamine is used in the manufacture of plastics, melamineformaldehyde resins; rubber, synthetic textiles; laminates, adhesives, and molding compoundКанцерогенность
A bioassay of melamine was conducted in rats and mice by NTP. Male F344 rats and B6C3F1 mice were administered melamine in their diets at concentrations of 2250 or 4500 ppm daily for 103 weeks.Female rats were fed 4500 or 9000 ppm melamine. At the end of 111 weeks, surviving animals were killed and examined.Методы очистки
Crystallise Melamine from water or dilute aqueous NaOH. It sublimes at ~240o on prolonged heating. [Beilstein 26 I 74, 26 II 132, 26 III/IV 1253.]Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Melamine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents such as hydrides, nitrides, alkali metals, and sulfides.Меламин запасные части и сырье
сырьё
1of2
запасной предмет
- Early-strength admixture
- water proofing agent 703
- softener PEG
- Fluorescent Brightener BC
- REACTIVE GREEN 19
- REACTIVE BLUE 4
- Various color amino baking enamel A04-9
- demulsifier KN-1
- modified phenol-formaldehyde resin
- Pumping agent
- Amino resin varnish
- finishing agent KB for polyester viscose blend
- anchorage used for glassine paper
- PAINT
- Меламиновая смола
1of5
Меламин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13637972665 +86-13637972665 |
China | 81 | 58 | ||
| +8613817748580 | China | 40066 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-18033737140 +86-17354101231 |
China | 227 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +8617756083858 | China | 973 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +16837774622 | China | 295 | 58 |