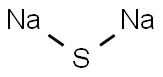
Натрия сульфид
- английское имяSodium sulfide
- CAS №1313-82-2
- CBNumberCB3429046
- ФормулаNa2S
- мольный вес78.04
- EINECS215-211-5
- номер MDLMFCD00003498
- файл Mol1313-82-2.mol
| Температура плавления | 950 °C(lit.) | ||||||||||||||
| плотность | 1.86 g/mL at 25 °C(lit.) | ||||||||||||||
| температура хранения | Refrigerator (+4°C) | ||||||||||||||
| растворимость | H2O: 0.1 g/mL, clear, colorless | ||||||||||||||
| форма | flakes | ||||||||||||||
| цвет | Yellow | ||||||||||||||
| Удельный вес | 1.856 | ||||||||||||||
| Запах | Rotten egg odor | ||||||||||||||
| Растворимость в воде | 186 g/L (20 ºC) | ||||||||||||||
| Чувствительный | Hygroscopic | ||||||||||||||
| Кристальная структура | Reverse CaF2 type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Мерк | 14,8681 | ||||||||||||||
| Space group | Fm3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Диэлектрическая постоянная | 5.0(Ambient) | ||||||||||||||
| Стабильность | Spontaneously flammable. Incompatible with acids, metals, oxidizing agents. Contact with acid liberates toxic gas. Fine dust/air mixtures are explosive. Hygroscopic. | ||||||||||||||
| ИнЧИКей | CXPWOVUZRAFMDA-UHFFFAOYSA-N | ||||||||||||||
| Справочник по базе данных CAS | 1313-82-2(CAS DataBase Reference) | ||||||||||||||
| Вещества, добавляемые в пищу (ранее EAFUS) | SODIUM SULFIDE | ||||||||||||||
| FDA 21 CFR | 172.615; 177.2600 | ||||||||||||||
| Рейтинг продуктов питания EWG | 3-5 | ||||||||||||||
| FDA UNII | YGR27ZW0Y7 | ||||||||||||||
| Справочник по химии NIST | Sodium sulfide(1313-82-2) | ||||||||||||||
| Система регистрации веществ EPA | Sodium sulfide (1313-82-2) | ||||||||||||||
| UNSPSC Code | 12352300 | ||||||||||||||
| NACRES | NA.23 |
| Коды опасности | C,N,Xn | |||||||||
| Заявления о рисках | 31-34-50-22 | |||||||||
| Заявления о безопасности | 26-45-61-36/37/39 | |||||||||
| РИДАДР | UN 1849 8/PG 2 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | WE1905000 | |||||||||
| F | 3-8-9-23 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.2 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28301010 | |||||||||
| Банк данных об опасных веществах | 1313-82-2(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H400:Чрезвычайно токсично для водных организмов.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H301+H311:Токсично при проглатывании или при контакте с кожей.
H290:Может вызывать коррозию металлов.
H251:Возможно самопроизвольное возгорание.
-
оператор предупредительных мер
P235:Держать в прохладном месте.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Натрия сульфид химические свойства, назначение, производство
Химические свойства
Sodium sulfide,Na2S, also known as sodium sulfuret,is an irritating, water-soluble, yellowish to reddish, deliquescent powder that melts at 1180°C (2156 °F). Sodium sulfide is used as a chemical intermediate and solvent,in conversion of wood into paper pulp, as a photographic and analytical reagent,as a source of sulfide,as a reducing agent,in organic reactions, as a depilatory, and in sheep dips.Физические свойства
White cubic crystal; hygroscopic; density 1.856 g/cm3; melts at 1,172°C; soluble in water 18.6 g/100mL at 20°C and 39 g/100mL at 50°C; aqueous solutions strongly alkaline; slightly soluble in alcohol; insoluble in ether.The pentahydrate consists of flat, shiny prismatic crystals; density 1.58 g/cm3; loses three water molecules at 100°C; melts at 120°C losing all water molecules; soluble in water and alcohol; aqueous solutions strongly alkaline; insoluble in ether.
The nonahydrate is a yellowish-white crystalline solid; tetragonal crystals; odor of hydrogen sulfide; the color changes on exposure to light and air, first turning to yellow and then becoming brownish-black, deliquescent; density 1.43 g/cm3; decomposes at about 50°C; very soluble in water; aqueous solution strongly alkaline; slightly soluble in alcohol; insoluble in ether.
Использование
These yellow flakes were made by fusing sodium carbonate with sulfur. Soluble in water but less so in alcohol, sodium sulfide was lovingly called “stink” by those who used it for toning prints or intensifying negatives because of its sulfurous smell.Подготовка
Sodium sulfide is prepared by heating sodium bisulfate with sodium chloride and coal above 950°C. The product mixture is extracted with water and the hydrated sulfide is obtained from the solution by crystallization: NaHSO4 + NaCl + 2C → Na2S + 2CO2↑ + HCl↑Sodium sulfide also is produced from its elements in liquid ammonia: Na + 2S → Na2S.
Определение
ChEBI: A sulfide salt with formula Na2S. The pentahydrate and (particularly) the nonahydrate are also known. In gel form, sodium sulfide is used to soften toenails to assist in trimming (and so relive pain) of ingrowing toenails.Общее описание
Sodium sulfide is a yellow to brick red crystalline mass or fused solid with an odor of rotten eggs. If exposed to moist air Sodium sulfide is liable to spontaneous heating and may cause ignition of nearby combustible material. Sodium sulfide absorbs moisture from the air.Реакции воздуха и воды
Aqueous solutions of sodium sulfide when exposed to air slowly convert to sodium hydroxide and sodium thiosulfate. The crystalline form upon exposure to air forms hydrogen sulfide and sodium carbonate [Merck 11th ed. 1989].Профиль реактивности
SODIUM SULFIDE is a white to yellow crystalline material, flammable. Can explode on rapid heating or when shocked. Violent reaction with carbon, charcoal, diazonium salts, N,N-dichloromethylamine, strong oxidizers, water. On contact with acids Sodium sulfide liberates highly toxic and flammable hydrogen sulfide gas. When heated to decomposition Sodium sulfide emits toxic fumes of sodium oxide, and oxides of sulfur [Bretherick, 5th ed., 1995, p. 1729].Опасность
Flammable, dangerous fire and explosion risk. Strong irritant to skin and tissue, liberates toxic hydrogen sulfide on contact with acids.Угроза здоровью
Caustic action on skin and eyes. If ingested may liberate hydrogen sulfide in stomach.Пожароопасность
Special Hazards of Combustion Products: Irritating sulfur dioxide is produced in fire.Промышленное использование
In non-metallic flotation, sodium sulfide is also used as a depressant and for collector desorption, in particular, fatty acids from monazite, pyrochlore, zircon and microcline. As a depressant for quartz, sodium sulfide is an excellent depressant for iron-activated quartz as well as non-activated quartz.Профиль безопасности
A poison by ingestion and intraperitoneal routes. Flammable when exposed to heat or flame. Unstable and can explode on rapid heating or percussion. Reacts violently with carbon, diazonium salts, n,n-dichloromethylamine, onitroaniline diazonium salt, water. When heated to decomposition it emits toxic fumes of SOx and Na2O. See also SULFIDESМетоды очистки
Some purification of the hydrated salt can be achieved by selecting large crystals and removing the surface layer (contaminated with oxidation products) by washing with distilled water. Other metal ions can be removed from Na2S solutions by passage through a column of Dowex ion-exchange A-1 resin, Na+-form. The hydrated salt can be rendered anhydrous by heating it in a stream of H2 or N2 until water is no longer evolved. (The resulting cake should not be heated to fusion because it is readily oxidised.) Recrystallise it from distilled water [Anderson & Azowlay J Chem Soc, Dalton Trans 469 1986]. Note that sodium sulfide hydrolyses in H2O to form NaHS + H2O, and is therefore alkaline. A 0.1N solution in H2O is 86% hydrolysed at room temperature. Its solubility in H2O is 8% at 0o, 12% at 20o and 30% at 50o. The anhydrous salt is obtained by allowing it to stand in a vacuum over conc H2SO4 or P2O5 at 45o to start with, then at 30-35o when the salt contains 4% of water. The last traces of water are removed by heating to 700o in a glass or porcelain tube in a stream of H2 to give pure H2S. [Fehér in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I pp 358-360 1963.]Натрия сульфид запасные части и сырье
сырьё
1of3
запасной предмет
- Prometryn
- AMIDO GREEN BLACK B
- REACTIVE GREEN 19
- Phosphorous acid chloride and phosphorus dichloride
- 4-аминобензальдегид
- 4-ацетамидобензальдегид
- Селена сульфид
- Direct Black 19
- Sulfur Red Brown B3R
- 4-Diazodiphenylamine sulfate
- 5-CHLORO-2-METHOXYANILINE HYDROCHLORIDE
- VARIAMINE BLUE B DIAZONIUM SALT
- Бис (2-нитрофенил) дисульфид
- 5-БРОМ-3-ХЛОР-2-ПИРИДИНОН
- Быстрый синий BB
- 3-Хлор-4-метиланилина
- 4-н-Butoxyaniline
- Calcium chlorate dihydrate
- O,O-dialkyl thiophosphoryl chloride
- 2-(4-МЕТИЛФЕНИЛ)-БЕНЗОТИАЗОЛ
1of8
Натрия сульфид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +8619931165850 | China | 1000 | 58 | |
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 |