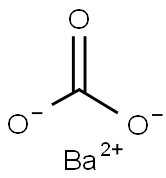
Бария карбонат
- английское имяBarium carbonate
- CAS №513-77-9
- CBNumberCB2145712
- ФормулаCBaO3
- мольный вес197.34
- EINECS208-167-3
- номер MDLMFCD00003448
- файл Mol513-77-9.mol
химическое свойство
| Температура плавления | 811 °C |
| Температура кипения | 1450 °C |
| плотность | 4.43 |
| Плотность накопления | 350kg/m3 |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | 0.02g/l |
| форма | Powder/Solid |
| цвет | White |
| Удельный вес | 4.43 |
| Запах | Odorless |
| РН | 7-8 (0.016g/l, H2O, 16℃) |
| Растворимость в воде | 0.002 g/100 mL (20 ºC) |
| Разложение | 1300°C |
| Мерк | 14,969 |
| БРН | 7045119 |
| Константа произведения растворимости (Ksp) | pKsp: 8.59 |
| Стабильность | Stable. Incompatible with strong acids. |
| Справочник по базе данных CAS | 513-77-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 6P669D8HQ8 |
| Система регистрации веществ EPA | Barium carbonate (513-77-9) |
| UNSPSC Code | 12352302 |
| NACRES | NA.23 |
больше
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22 | |||||||||
| Заявления о безопасности | 24/25 | |||||||||
| РИДАДР | UN 1564 6.1/PG 3 | |||||||||
| WGK Германия | - | |||||||||
| RTECS | CQ8600000 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 6.1(b) | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28366000 | |||||||||
| Банк данных об опасных веществах | 513-77-9(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 418 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Бария карбонат химические свойства, назначение, производство
Описание
Barium carbonate has the molecular formula of BaCO3 and the molecular weight of 197.3359 g/mol. Its CAS number is 513-77-9. Barium carbonate has only one stable form (aragonite-type structure) and temperature of precipitation has no effect on crystal form, unlike that of calcium or magnesium carbonates.Химические свойства
Barium carbonate, BaCO3, also known as witherite, is a white powder that is soluble in acids,with the exception of sulfuric acid.It has a melting point of 174°C and is used in television picture tubes, rodenticide, optical glass and ceramic flux.
Barium oxide, BaO, is manufactured by decomposition of barium carbonate.
Физические свойства
White powder; orthorhombic crystal system; density 4.286 g/cm3; refractive index 1.60; hardness 3.50 Mohs; melts at 811°C; insoluble in water (c. 25 mg/L at 25°C); Ksp 2.0 x 10-9.Вхождение
Barium carbonate is found in nature as mineral witherite. The compound has many major commercial applications in brick, glass, ceramics, oil-drilling, photographic and chemical industries. It is mixed with wet clay to immobilize many water-soluble salts in making uniform red bricks. In the glass industry, barium is added to glass as barium carbonate or barium oxide to improve the refractive index of optical glass; also to promote sintering and lower the viscosity of melted glass to make glass bead formation easy. It is used in the manufacture of television picture tubes and photographic paper. Another important application involves its use as a fluxing ingredient in ceramic industry for enamels, glazes and ceramic bodies. Barium carbonate is used in oil-well drilling to insolubilize gypsum and inhibit coagulation; in ferrous metallurgy for steel carburizing; in chlor-alkali cells for treating salt brines to remove sulfates; and to make ferrite, and barium titanate. Many barium salts are prepared from barium carbonate.Использование
Barium carbonate has many major commercial applications in brick, glass, ceramics, oil-drilling, photographic and chemical industries. It is mixed with wet clay to immobilize many water-soluble salts in making uniform red bricks. In the glass industry, barium is added to glass as barium carbonate or barium oxide to improve the refractive index of optical glass and also to promote sintering and lower the viscosity of melted glass to make glass bead formation easy. It is used in the manufacture of television picture tubes and photographic paper. Another important application involves its use as a fluxing ingredient in ceramic industry for enamels, glazes and ceramic bodies. Barium carbonate is used in oil well drilling to insolubilize gypsum and inhibit coagulation; in ferrous metallurgy for steel carburizing; in chloralkali cells for treating salt brines to remove sulfates; and to make ferrites, and barium titanate. Many barium salts are prepared from barium carbonate.Определение
barium carbonate: A white insolublecompound, BaCO3; r.d. 4.43. It decomposeson heating to give bariumoxide and carbon dioxide:BaCO3(s) → BaO(s) + CO2(g)
The compound occurs naturally asthe mineral witherite and can be preparedby adding an alkaline solutionof a carbonate to a solution of a bariumsalt. It is used as a raw materialfor making other barium salts, as aflux for ceramics, and as a raw materialin the manufacture of certaintypes of optical glass.
Подготовка
Barium carbonate is made commercially from barium sulfide either by treatment with sodium carbonate or ammonium carbonate at 60 to 70°C or by passing CO2 gas through a soluble Ba2+ solution at 40 to 90°C.Общее описание
Barium carbonate is a white powder. Barium carbonate is insoluble in water and soluble in most acids, with the exception of sulfuric acid. Barium carbonate has a specific gravity of 4.275. Barium carbonate is toxic by ingestion.Реакции воздуха и воды
Barium carbonate is insoluble in water and soluble in most acids, with the exception of sulfuric acid.Профиль реактивности
Salts, basic, such as Barium carbonate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.Опасность
A poison.Угроза здоровью
(INGESTION ONLY): excessive salivation, vomiting, severe abdominal pain, and violent purging with watery and bloody stools; a slow and often irregular pulse and a transient elevation in arterial blood pressure; tinnitus, giddiness and vertigo; muscle twitchings, progressing to convulsions and/or paralysis; dilated pupils with impaired accommodation; confusion and increasing somnolence, without coma; collapse and death from respiratory failure and cardiac arrest.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Профиль безопасности
Poison by ingestion, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: stomach ulcers, muscle weakness, paresthesias and paralysis, hypermotility, diarrhea, nausea or vomiting, lung changes. Experimental reproductive effects. Incompatible with BrF3 and 2- furanpercarboxylic acid. See also BARIUM COMPOUNDS (soluble).Бария карбонат запасные части и сырье
сырьё
1of4
запасной предмет
- Бария титанат(IV)
- Никеля(II) хлорид гексагидра
- Бария октагидрат гидроксид
- High temperature shift calalyst
- Manganous dihydrogen phosphate
- Барий азотистокислый
- Бария нитра
- Sodium tripolyphosphate
- Бария хлорида дигидра
- Моногидрат гидроксида бария
1of4
Бария карбонат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-13862580743 +86-13862580743 |
China | 71 | 58 | ||
| +86-13637972665 +86-13637972665 |
China | 81 | 58 | ||
| +86-86-17798073498 +8617798073498 |
China | 299 | 58 | ||
| +8618601192704 | China | 76 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | ||
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +8613288715578 | China | 1174 | 58 |