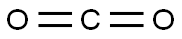
УГЛЕКИСЛЫЙ ГАЗ
- английское имяCarbon dioxide
- CAS №124-38-9
- CBNumberCB5778186
- ФормулаCO2
- мольный вес44.01
- EINECS204-696-9
- номер MDLMFCD00011491
- файл Mol124-38-9.mol
| Температура плавления | −78.5 °C(lit.) |
| Температура кипения | -78.46°C |
| плотность | 1.977(0℃) |
| плотность пара | 1.52 (vs air) |
| давление пара | 56.5 atm ( 20 °C) |
| показатель преломления | 1.0004 |
| температура хранения | −70°C |
| растворимость | At 20 °C and at a pressure of 101 kPa, 1 volume dissolves in about 1 volume of water. |
| форма | colorless gas |
| цвет | colorless |
| Запах | at 100.00?%. odorless |
| Растворимость в воде | mL CO2/100mL H2O at 760mm: 171 (0°C), 88 (20°C), 36 (60°C) [MER06] |
| Мерк | 13,1819 |
| БРН | 1900390 |
| Пределы воздействия | TLV-TWA 5000 ppm (~9000 mg/m3) (ACGIH, MSHA, and OSHA); STEL 30,000 ppm (ACGIH). |
| Диэлектрическая постоянная | 1.6(0℃) |
| Стабильность | Stable. Incompatible with chemically active metals, such as alkali metals. |
| LogP | 0.830 (est) |
| Справочник по базе данных CAS | 124-38-9(CAS DataBase Reference) |
| FDA 21 CFR | 184.1240; 582.1240 |
| Вещества, добавляемые в пищу (ранее EAFUS) | CARBON DIOXIDE |
| Специальный комитет по веществам GRAS | Carbon dioxide |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 142M471B3J |
| Словарь онкологических терминов NCI | carbon dioxide |
| Код УВД | V03AN02 |
| Система регистрации веществ EPA | Carbon dioxide (124-38-9) |
| UNSPSC Code | 12352119 |
| NACRES | NA.22 |
| Заявления о безопасности | 9 | |||||||||
| РИДАДР | UN 1013 2.2 | |||||||||
| OEB | A | |||||||||
| OEL | TWA: 5000 ppm (9000 mg/m3), STEL: 30,000 ppm (54,000 mg/m3) | |||||||||
| WGK Германия | - | |||||||||
| RTECS | FF6400000 | |||||||||
| F | 4.5-31 | |||||||||
| Классификация DOT | 2.2 (Non-flammable gas) | |||||||||
| Класс опасности | 2.2 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28112100 | |||||||||
| Банк данных об опасных веществах | 124-38-9(Hazardous Substances Data) | |||||||||
| ИДЛА | 40,000 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H280:Газ под давлением. Баллоны (емкости) могут взрываться при нагревании.
-
оператор предупредительных мер
P410+P403:Беречь от солнечных лучей. Хранить в хорошо вентилируемом месте.
УГЛЕКИСЛЫЙ ГАЗ химические свойства, назначение, производство
Описание
Carbon dioxide is a colorless, odorless, noncombustible gas present throughout the atmosphere and is an essential compound for life on Earth. It is found on other planets in the solar system. Mars’s icecaps are primarily frozen carbon dioxide and Venus’s atmosphere is mostly carbon dioxide. Carbon dioxide occurs naturally as approximately 0.03% v/v of the atmosphere.Solid carbon dioxide, also known as dry ice, is usually encountered as white-colored pellets or blocks.Химические свойства
Carbon dioxide,CO2, also known as carbonic anhydride and carbonic acid gas, is a colorless,odorless gas that liquifies at -65 °C(-86 OF) and solidifies in dry ice at -78.2 °C(-107 OF). It is soluble in water,alcohol, and most alkaline solutions. In a relatively slow reaction,carbon dioxide hydrates in water to become carbonic acid and is corrosive. In petroleum production, the velocity of the carbon dioxide gas can increase the corrosion rate to very high levels,with the presence of salts becoming unimportant. Carbon dioxide is used in preparing carbonated beverages, fire extinguishers, dry ice refrigerants,and as a raw material in the production of sodium carbonate and sodium bicarbonate using the Solvay procedure.Физические свойства
Colorless, odorless and tasteless gas; 1.53 times heavier than air; density 1.80 g/L at 25°C; can be liquefied under pressure; liquefies at -56.6°C at 5.2 atm; density of liquid CO2 at 0°C and 34 atm 0.914 g/mL; solidifies to white snow-like flakes known as dry ice, density 1.56 g/cm3 at -79°C; dry ice sub limes to CO2 gas at -78.5°C; critical temperature 31°C; critical pressure 72.79 atm, critical density 94 cm3/mol; moderately soluble in water, solubility 173 mL and 88mL CO2/100 mL water at 0°C and 20°C, respectively; solubility increases with pressure.История
The discovery of carbon dioxide, credited to Joseph Black (1728–1799), played a critical role in supplanting the phlogiston theory and advancing the development of modern chemistry. Black, in his medical studies, was searching for a substance to dissolve kidney stones, but he switched his subject to a study of stomach acidity. Black was working with the carbonates magnesia alba (magnesium carbonate) and calcium carbonate (limestone) and observed that when magnesia alba was heated or reacted with acids, it produced a gas and a salt. Black, who published his work in 1756, called the gas “fixed air” and noted that it had properties similar to those described by Jan Baptista van Helmont (1577–1644) for spiritus sylvestrius. Spiritus sylvestrius was the gas produced during combustion processes, and van Helmont realized that this was the same gas produced during fermentation and when acids reacted with seashells.Использование
Carbon Dioxide is a gas obtained during fermentation of glucose (grain sugar) to ethyl alcohol. it is used in pressure-packed foods as a propellant or aerating agent and is also used in the carbonation of beverages. it is released as a result of the acid carbonate reaction of leavening agents in baked goods to produce an increase in volume. as a solid, it is termed dry ice and is used for freezing and chilling.Определение
1. The solution of carbon dioxide in a liquid under pressure, as in carbonated soft drinks.2. The addition of carbon dioxide to compounds, e.g. the insertion of carbon dioxide into Grignard reagents.
Методы производства
Carbon dioxide is obtained industrially in large quantities as a byproduct in the manufacture of lime; by the incineration of coke or other carbonaceous material; and by the fermentation of glucose by yeast. In the laboratory it may be prepared by dropping acid on a carbonate.Подготовка
Carbon dioxide is produced as a by-product in many processes. It is pro duced as a by-product in the manufacture of lime from calcium carbonate:
CaCO3 →CaO + CO2
CO2 also is derived from synthesis gas which is a mixture of CO, CO2, H2 and N2 from air obtained by steam reforming. Carbon dioxide also is obtained by combustion of natural gas:
CH4 + 2O2 → CO2 + 2H2O
It also is obtained as a by-product in the Haber-Bosch process for the man ufacture of ammonia. The method involves passing steam and air over hot coke.
Carbon dioxide also is produced along with ethanol from fermentation of carbohydrates by yeast:
C6H12O6→2CO2 + 2C2H5OH
In the laboratory, CO2 may be produced by the reaction of any carbonate with a dilute mineral acid:
CaCO3 + 2HCl → CaCl2 + CO2 + H2O
Общее описание
An odorless, white solid. Can cause damaging frostbite. Noncombustible and nontoxic. Liquefies at -109°F. Can asphyxiate by displacement of air. Used as a refrigerant.Реакции воздуха и воды
Water soluble. Forms carbonic acid, a mild acid in water.Профиль реактивности
Contact of very cold liquid/solid carbon dioxide with water may result in vigorous or violent boiling of the product and extremely rapid vaporization due to the large temperature differences involved. If the water is hot, there is the possibility that a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if liquid gas contacts water in a closed container. With water forms weak carbonic acid in nonhazardous reaction. Dusts of magnesium, lithium, potassium, sodium, zirconium, titanium, and some magnesium-aluminum alloys, and heated aluminum, chromium, and magnesium when suspended in carbon dioxide are ignitable and explosive. This is especially true in the presence of strong oxidizers, such as peroxides. The presence of carbon dioxide in solutions of aluminum hydride in ether can cause violent decomposition on warming the residue, [J. Amer. Chem. Soc., 1948, 70, 877]. Dangers arising from the use of carbon dioxide in the fire prevention and extinguishing systems of confined volumes of air and flammable vapors are examined. The hazard associated with its use centers around the fact that large electrostatic discharges may be created that initiate explosion, [Quart. Saf. Summ., 1973, 44(1740, 10].Опасность
Solid damaging to skin and tissue; keep away from mouth and eyes. Asphyxia.Угроза здоровью
Carbon dioxide is an asphyxiant. Exposureto about 9–10% concentration can causeunconsciousness in 5 minutes. Inhalation of3% CO2 can produce weak narcotic effects.Exposure to 2% concentration for severalhours can produce headache, increased bloodpressure, and deep respiration. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite.Пожароопасность
Non-flammable gases. Containers may explode when heated. Ruptured cylinders may rocket.Сельскохозяйственное использование
Solid carbon dioxide is known as dry ice. It is produced by cooling gaseous carbon dioxide under pressure.Dry ice is used in refrigeration, carbonated drinks and fire extinguishers. It is also a constituent of medical gases, as it promotes exhalation.
Профиль безопасности
An asphpant. See discussion of simple asphyxiants under ARGON. Experimental teratogenic and reproductive effects. Contact of solid carbon dioxide snow with the skin can cause burns. Dusts of magnesium, zirconium, titanium, and some magnesium-aluminum alloys igmte and then explode in COa atmospheres. Dusts of aluminum, chromium, and manganese ignite and then explode when heated in CO2. Several bulk metals wlll burn in CO2. Reacts vigorously with (Al + Na2O2), Cs2O, Mg(C2H5)2, Li, (Mg + Na2O2), K, KHC, Na, Na2C2, NaK, Ti. CO2 fire extingushers can produce highly incendiary sparks of 5-1 5 mJ at 10-20 kV by electrostatic discharge. Incompatible with acrylaldehyde, aziridme, metal acetylides, sodium peroxide.Безопасность
In formulations, carbon dioxide is generally regarded as an essentially nontoxic material.Возможный контакт
Gaseous Carbon dioxide is used to carbonate beverages; as a weak acid in the textile, leather, and chemical industries; in water treatment; and in the manufacture of aspirin and white lead; for hardening molds in foundries; in food preservation, in purging tanks and pipe lines; as a fire extinguisher, in foams; and in welding. Because it is relatively inert, it is utilized as a pressure medium. It is also used as a propellant in aerosols; to promote plant growth in green houses; it is used medically as a respiratory stimulant; in the manufacture of carbonates; and to produce an inert atmosphere when an explosive or flammable hazard exists. The liquid is used in fire extinguishing equipment; in cylinders for inflating life rafts; in the manufacturing of dry ice, and as a refrigerant. Dry ice is used primarily as a refrigerant. Occupational exposure to carbon dioxide may also occur in any place where fermentation processes may deplete oxygen with the formation of carbon dioxide, e.g., in mines, silos, wells, vats, ships’ holds, etc.Экологическая судьба
Carbon dioxide is an asphyxiant, means it causes toxicity by displacing oxygen from the breathing atmosphere primarily in enclosed spaces or in open spaces due to sudden release of massive amounts of CO2 (for example, forests fire or natural emission during a volcanic eruption) and results in hypoxia. Thehumanbody produces about 12 000–13 000 mmols per day of CO2 and is excreted primarily via lungs. The CO2 concentration in plasma is maintained within a narrow range of 40±5 mm Hg (4.7–6 KPa). At plasma concentration of 22.5mmHg (3 KPa) or less death can occur within few minutes. The cause of death in breathing high concentration ofCO2 is due to CO2 poisoning, that results in rapid decrease in blood pH (respiratory acidosis,хранилище
Extremely stable and chemically nonreactive. Store in a tightly sealed cylinder. Avoid exposure to excessive heat.Перевозки
Carbon dioxide (UN1013, UN2187), Hazard Class: 2.2; Labels: 2.2-Nonflammable compressed gas. Dry ice (UN1845), Hazard class 9 is considered a “miscellaneous hazardous material” and does not require a label. The gas and refrigerated liquid fall in Hazard Class 2.2 and there is no Packing Group; solid, dry ice falls in Hazard Class 9. Solid, dry ice carries the symbol “AW.” The letter “A” restricts the application of requirements of this subchapter to materials offered or intended for transportation by aircraft, unless the material is a hazardous substance or a hazardous waste. The letter “W” restricts the application of requirements of this subchapter to materials offered or intended for transportation by vessel, unless the material is a hazardous substance or a hazardous waste. Cylindersmust be transported in a secure upright position, in a wellventilated truck. Protect cylinder and labels from physical damage. The owner of the compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas cylinders without the express written permission of the owner.Методы очистки
Pass the gas over CuO wire at 800o to oxidise CO and other reducing impurities (such as H2), then over copper dispersed on Kieselguhr at 180o to remove oxygen. Drying it at -78o removes the water vapour. Final purification is by vacuum distillation at liquid nitrogen temperature to remove non-condensable gases [Anderson et al. J Chem Soc 3498 1962]. Sulfur dioxide contaminant can be removed at 450o using silver wool combined with a plug of platinised quartz wool. Halogens are removed by using Mg, Zn or Cu, heated to 450o. [Glemsner in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 647 1963.]Утилизация отходов
Return refillable compressed gas cylinders to supplier. Vent to atmosphereРегуляторный статус
GRAS listed. Accepted for use in Europe as a food additive. Included in the FDA Inactive Ingredients Database (aerosol formulation for nasal preparations; IM and IV injections). Included in the Canadian List of Acceptable Non-medicinal Ingredients.УГЛЕКИСЛЫЙ ГАЗ запасные части и сырье
сырьё
1of2
запасной предмет
- 1-метил-4-пиперидинола гидрохлорид
- 4-трет-Butylcyclohexanecarboxylic кислота
- 3,6-DICHLORO-2-HYDROXY BENZOIC ACID
- 3-нитрофенилуксусная кислота
- 3,3,3-Trifluoropropionic кислота
- Этил 4-пиридилацета
- 6-CHLORO-2-(TRIFLUOROMETHYL)QUINOLINE-4-CARBOXYLIC ACID
- 4-НИТРОЗОДИФЕНИЛАМИН
- Zirconium carbonate oxide
- Тиофен-2-сульфонилхлорид
- 1-Chlorophthalazine
- 2-(Трифторметил)бензойная кислота
- Литий дигидрата ацетата
- 2,3,6-Трифторбензойная кислота
- DL-лейцин
- 2,5-дихлоризоникотиновая кислота
- 2,4,6-трифторбензойной кислоты
- Магния ацетат тетрагидра
- 2-Bromo-6-fluorobenzoic acid
- N-(4-хлорфенил)-2-гидрокси-9H-карбазол-3-карбоксамид
- Ultrafine calcium carbonate
- 1-гидрокси-2-нафтойная кислота
- 2,4,6-Trichlorobenzoic кислота
- 2,3,5,6-ТЕТРАФТОРО-4-МЕТИЛБЕНЗОЙНАЯ КИСЛОТА
- 1,3-тиазол-2-карбоновая кислота
- ХЛОРИД БРОМА
- 1,3-тиазол-2-карбонилхлорид
- Циннамоил хлорид
- Хлорацетальдегид диэтил ацетал
- 1-метилпиррол-2-карбоновой кислоты
1of8
УГЛЕКИСЛЫЙ ГАЗ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-0519-85551759 +8613506123987 |
China | 8840 | 58 | |
| +86-13806087780 | China | 17365 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 30233 | 58 | |
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | |
| +86-29-89586680 +86-15129568250 |
China | 22787 | 58 | |
| +86-25-58227606 +86-15305155328 |
China | 4128 | 58 | |
| +8617732866630 | China | 18147 | 58 |