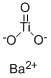
Бария титанат(IV)
- английское имяBarium titanate
- CAS №12047-27-7
- CBNumberCB0265773
- ФормулаBaO3Ti
- мольный вес233.19
- EINECS234-975-0
- номер MDLMFCD00003447
- файл Mol12047-27-7.mol
| Температура плавления | 1625 °C | ||||||||||||||
| плотность | 6.08 g/mL at 25 °C(lit.) | ||||||||||||||
| Плотность накопления | 1400kg/m3 | ||||||||||||||
| давление пара | 0Pa at 20℃ | ||||||||||||||
| температура хранения | no restrictions. | ||||||||||||||
| растворимость | Soluble in alcohols | ||||||||||||||
| форма | powder | ||||||||||||||
| цвет | White to gray | ||||||||||||||
| Удельный вес | 6.08 | ||||||||||||||
| РН | 9.6 (20g/l, H2O, 25℃)(slurry) | ||||||||||||||
| Растворимость в воде | INSOLUBLE | ||||||||||||||
| Мерк | 14,1000 | ||||||||||||||
| crystal system | square | ||||||||||||||
| Space group | P4mm | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| ИнЧИКей | WNKMTAQXMLAYHX-UHFFFAOYSA-N | ||||||||||||||
| LogP | 1 at 20℃ | ||||||||||||||
| Справочник по базе данных CAS | 12047-27-7(CAS DataBase Reference) | ||||||||||||||
| FDA UNII | 73LKE302QO | ||||||||||||||
| Система регистрации веществ EPA | Barium titanium oxide (BaTiO3) (12047-27-7) | ||||||||||||||
| UNSPSC Code | 12352303 | ||||||||||||||
| NACRES | NA.23 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 20/22 | |||||||||
| Заявления о безопасности | 28-28A | |||||||||
| РИДАДР | UN 1564 6.1/PG 3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | XR1437333 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28419085 | |||||||||
| Токсичность | rat,LD50,intraperitoneal,3gm/kg (3000mg/kg),GASTROINTESTINAL: OTHER CHANGESBLOOD: HEMORRHAGEKIDNEY, URETER, AND BLADDER: OTHER CHANGES,Industrial Medicine and Surgery. Vol. 31, Pg. 302, 1962. | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302+H332:Вредно при проглатывании или при вдыхании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
Бария титанат(IV) химические свойства, назначение, производство
Описание
Attributing to its ferroelectricity, barium titanate is applied as a semiconductor with positive temperature coefficient of resistivity. It is one of the most important electroceramic materials among all the ferroelectric materials. Barium titanate has also dielectric properties, which can be used in the manufacture of ceramic capacitors. Furthermore, barium titanate has piezoelectric property and can be used in electrical devices such as actuators, accelerators, ultrasonic generators, piezoelectric transducers, filters, and sensors.Химические свойства
White crystalline solid; exists in five crystal modifications; the common tetragonal form has a Curie point of 120°C; exhibits ferroelectric and piezoelectric properties; density 6.02 g/cm3; melts at 1,625°C; insoluble in water and alkalies; slightly soluble in dilute mineral acids; dissolves in concentrated sulfuric acid and hydrofluoric acid.Физические свойства
White crystalline solid; exists in five crystal modifications; the common 94 BARIUM TITANATE tetragonal form has a Curie point of 120°C; exhibits ferroelectric and piezoelectric properties; density 6.02 g/cm3; melts at 1,625°C; insoluble in water and alkalies; slightly soluble in dilute mineral acids; dissolves in concentrated sulfuric acid and hydrofluoric acid.Физические свойства
Energy Gap:Eg=3:38 eV(E//c); 3:27 eV(E⊥c).
The energy gap decreases by -4.5×10-4 eV/℃ when the crystal structure becomes cubic at high temperature.
Dielectric Constants:
Saturated Polarizability: 78,000 esu, Dipole Moment: 5×10-18 esu cm-3 .
Использование
Barium titanate has many important commercial applications. It has both ferroelectric and piezoelectric properties. Also, it has a very high dielectric constant (about 1,000 times that of water). The compound has five crystalline modifications, each of which is stable over a particular temperature range. Ceramic bodies of barium titanate find wide applications in dielectric amplifiers, magnetic amplifiers, and capacitors. These storage devices are used in digital calculators, radio and television sets, ultrasonic apparatus, crystal microphone and telephone, sonar equipment, and many other electronic devices.Подготовка
Barium titanate is made by sintering a finely powdered mixture of barium carbonate and titanium dioxide in a furnace at 1,350°C. The calcined mass is finely ground and mixed with a binder (plastic). The mixture is subjected to extrusion, pressing or film casting to obtain ceramic bodies of desired shapes. Plastic is burnt off by heating and the shaped body is sintered by firing and then polished.Barium titanate also may be prepared by other methods. These include ignition of barium and titanium alcoholates in an organic solvent; treatment of tetraethyl titanate or other alkyl ester of titanium with an aqueous solution of barium hydroxide; and ignition of barium titanyloxalate.
Общее описание
Barium titanate(IV) is a ceramic material that can be synthesized by reacting titanium oxide (TiO2) and barium salts in the presence of oleic acid as a stabilizing agent. It has a cubic crystal structure which allows it to have ferroelectric, thermoelectric and piezoelectric properties. It forms a film with intense photoluminescence which can be used for a wide range of electronic applications.Структура и конформация
The space lattice of BaTiO 3 takes four types of crystal systems, depending on temperatures. The trigonal crystal (lower than -90℃) shows the spontaneous polarization to the [111] direction.The rhombic crystal (-70℃ to 5℃) shows the spontaneous polarization to the [011] direction and takes a longer structure along the direction. The tetragonal crystal (5℃–120℃) shows the spontaneous polarization to the [001] direction and takes a longer structure along the direction by the ratio of c/a=1.01. The cubic crystal (higher temperature than Curie point 120℃) takes the calcium titanate structure (Perovskite structure) and Ba atoms position at the eight corners of the cube, six O atoms at the center of the planes, and a Ti atom at the center of the body.использованная литература
[1] Burcu Ertu?, The Overview of The Electrical Properties of Barium Titanate, American Journal of Engineering Research, 2013, vol. 02, 01-07[2] G. Arlt, D. Hennings and G. de With, Dielectric properties of fine‐grained barium titanate ceramics, Journal of Applied Physics, 1985, vol. 58, 1619
[3] Tomoaki Karaki, Kang Yan and Masatoshi Adachi, Barium Titanate Piezoelectric Ceramics Manufactured by Two-Step Sintering, Japanese Journal of Applied Physics, 2007, vol. 46, 7035-7038
Бария титанат(IV) запасные части и сырье
Бария титанат(IV) поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 532-83657313 18663969289; |
China | 227 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-0311-87836622 +86-17333973358 |
China | 8051 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +86-18633929156 +86-18633929156 |
China | 972 | 58 | ||
| +8613288715578 | China | 1174 | 58 |