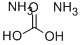
Аммония карбонат
- английское имяAmmonium carbonate
- CAS №506-87-6
- CBNumberCB9393644
- ФормулаCH8N2O3
- мольный вес96.09
- EINECS208-058-0
- номер MDLMFCD00010890
- файл Mol506-87-6.mol
химическое свойство
| Температура плавления | 58 °C |
| Температура кипения | 179.58°C (rough estimate) |
| плотность | 1.5 |
| плотность пара | 2.7 (vs air) |
| давление пара | 760 mm Hg @ 600C |
| показатель преломления | 1.4616 (estimate) |
| температура хранения | Store at RT. |
| растворимость | H2O: 0.1 g/mL at 25 °C, clear, colorless |
| форма | Solid |
| цвет | White to yellow |
| Запах | strong odor of NH3 |
| Водородный показатель | 9 |
| Растворимость в воде | SOLUBLE |
| Чувствительный | Hygroscopic |
| Мерк | 14,508 |
| БРН | 3627235 |
| LogP | -0.809 (est) |
| Справочник по базе данных CAS | 506-87-6(CAS DataBase Reference) |
| FDA 21 CFR | 184.1137; 582.1137 |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | PDP691CN28 |
| Система регистрации веществ EPA | Ammonium carbonate (506-87-6) |
| UNSPSC Code | 12352301 |
| NACRES | NA.55 |
больше
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-52/53 | |||||||||
| Заявления о безопасности | 24/25 | |||||||||
| РИДАДР | UN 9084 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | BP1925000 | |||||||||
| F | 13 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28369940 | |||||||||
| Банк данных об опасных веществах | 506-87-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 ivn-mus: 96 mg/kg AJVRAH 29,897,68 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P301+P312+P330:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии. Прополоскать рот.
Аммония карбонат химические свойства, назначение, производство
Химические свойства
Anunonium carbonate is a white water-soluble, volatile solid prepared by reaction of NH4OH and CO2 and crystallizing from dilute alcohol. Ammonium carbonate loses NH3,CO2, and H20 at ordinary temperatures, and rapidly at 58°C.Физические свойства
Colorless or translucent hard crystalline mass or white cubic crystals or powder; sharp taste; odor of ammonia; decomposes at 58°C; slow decomposition at ambient temperatures; readily dissolves in cold water; decomposes in hot water; insoluble in liquid ammonia, alcohol and carbon disulfide.Использование
Ammonium Carbonate is a dough strengthener, a leavening agent, a ph control agent, and a texturizer. it is prepared by the sublima- tion of a mixture of ammonium sulfate and calcium carbonate, and occurs as a white powder or a hard, white translucent mass.Подготовка
Ammonium carbonate is obtained by passing carbon dioxide into aqueous ammonia solution in a column or tower. Ammonia, carbon dioxide and water vapor are distilled and the vapors condensed into a solid crystalline mass. It also may be prepared by subliming a mixture of ammonium sulfate and calcium carbonate.Определение
ammonium carbonate: A colourlessor white crystalline solid,(NH4)2CO3, usually encountered asthe monohydrate. It is very soluble incold water. The compound decomposesslowly to give ammonia, water,and carbon dioxide. Commercial ‘ammoniumcarbonate’ is a double saltof ammonium hydrogencarbonateand ammonium aminomethanoate(carbamate), NH4HCO3.NH2COONH4.This material is manufactured byheating a mixture of ammoniumchloride and calcium carbonate andrecovering the product as a sublimedsolid. It readily releases ammoniaand is the basis of sal volatile. It isalso used in dyeing and wool preparationand in baking powders.Общее описание
A colorless crystalline solid or a white powder with a strong odor of ammonia. Noncombustible. The primary hazard is the threat to the environment. Immediate steps should be taken to limit spread to the environment. Used to make other ammonium compounds, in pharmaceuticals, in food processing.Реакции воздуха и воды
Water soluble.Профиль реактивности
Ammonium carbonate decomposes when heated to give gaseous ammonia and gaseous carbon dioxide. Reaction is non-explosvie. Causes decomposition of sodium hypochlorite within a few seconds [Mellor 2 Supp. 1:550 1956].Опасность
Evolves irritating fumes when heated.Угроза здоровью
Inhalation causes irritation of nose and throat. Ingestion may cause gastric irritation. Contact with eyes or skin causes irritation.Сельскохозяйственное использование
Ammonium carbonate, (NH4)2CO3, is an intermediate product formed during the synthesis of urea. Ammonium carbonate on decomposition yields urea and water.Профиль безопасности
Poison by subcutaneous and intravenous routes. When heated to decomposition it emits toxic fumes of NO, and NH3.Возможный контакт
It is used in dyeing, tanning, medicines, fire extinguishers; to make casein glue; ammonia salts; and baking powders. A laboratory reagent.Перевозки
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Несовместимости
Acids, acid salts; salts of iron and zinc, alkaloids, calomel and tartar emetic. Keep cool, below 38 C. Contact with inorganic acids may form CO2, heat, and dangerous spattering.Утилизация отходов
Slowly deposit in a large container of water. Add excess amounts of soda ash and let stand for 24 hours. Decant to another container, neutralize with hydrochloric acid, and drain with an excess of water. Ship to landfill.Аммония карбонат запасные части и сырье
сырьё
запасной предмет
1of5
Аммония карбонат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-13131129325 | China | 5887 | 58 | |
| +8617732866630 | China | 18147 | 58 | |
| +86-16264648883 +86-16264648883 |
China | 3712 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 18017610038 | CHINA | 3619 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86 319 5273535 | CHINA | 287 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| 18853181302 | CHINA | 5906 | 58 |