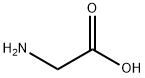
Глицин
- английское имяGlycine
- CAS №56-40-6
- CBNumberCB5336487
- ФормулаC2H5NO2
- мольный вес75.07
- EINECS200-272-2
- номер MDLMFCD00008131
- файл Mol56-40-6.mol
| Температура плавления | 240 °C (dec.) (lit.) |
| Температура кипения | 233°C |
| плотность | 1.595 |
| Плотность накопления | 920kg/m3 |
| давление пара | 0.0000171 Pa (25 °C) |
| показатель преломления | 1.4264 (estimate) |
| FEMA | 3287 | GLYCINE |
| Fp | 176.67°C |
| температура хранения | 2-8°C |
| растворимость | H2O: 100 mg/mL |
| форма | powder |
| пка | 2.35(at 25℃) |
| цвет | <5 (200 mg/mL)(APHA) |
| РН | 4(0.2 molar aqueous solution) |
| Запах | Odorless |
| Водородный показатель | 4 |
| Биологические источники | synthetic |
| Odor Type | odorless |
| Растворимость в воде | 25 g/100 mL (25 ºC) |
| λмакс | λ: 260 nm Amax: 0.05 λ: 280 nm Amax: 0.05 |
| Номер JECFA | 1421 |
| Мерк | 14,4491 |
| БРН | 635782 |
| Стабильность | Stable. Combustible. Incompatible with strong oxidizing agents. |
| ИнЧИКей | DHMQDGOQFOQNFH-UHFFFAOYSA-N |
| LogP | -3.21 |
| FDA 21 CFR | 172.812; 582.5049; 172.320; 310.545 |
| Вещества, добавляемые в пищу (ранее EAFUS) | GLYCINE |
| Справочник по базе данных CAS | 56-40-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | TE7660XO1C |
| Словарь наркотиков NCI | glycine |
| Код УВД | B05CX03 |
| Справочник по химии NIST | Glycine(56-40-6) |
| Система регистрации веществ EPA | Glycine (56-40-6) |
| Поглощение | ≤0.15 at 280 at 1M |
| UNSPSC Code | 41116107 |
| NACRES | NC.01 |
| Заявления о рисках | 33 | |||||||||
| Заявления о безопасности | 22-24/25 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | MB7600000 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29224910 | |||||||||
| Банк данных об опасных веществах | 56-40-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 7930 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P271:Использовать только на открытом воздухе или в хорошо вентилируемом помещении.
P280:Использовать перчатки/ средства защиты глаз/ лица.
Глицин химические свойства, назначение, производство
Описание
Glycine (abbreviated as Gly or G) is an organic compound with the formula NH2CH2COOH. Having a hydrogen substituent as its side-chain, glycine is the smallest of the 20 amino acids commonly found in proteins. Its codons are GGU, GGC, GGA, GGG of the genetic code.Glycine is a colourless, sweet-tasting crystalline solid. It is unique among the proteinogenic amino acids in that it is not chiral. It can fit into hydrophilic or hydrophobic environments, due to its minimal side chain of only one hydrogen atom. Glycine is also the genus name of the Soybean plant (species name = Glycine max).
Химические свойства
Glycine occurs as a white, odorless, crystalline powder, and has a sweet taste.Вхождение
Gelatin and silk fbroin are reportedly the best natural sources of this amino acidИспользование
Glycine is a non-essential amino acid for human development. Glycine is an inhibitory neurotransmitter in spinal cord, allosteric regulator of NMDA receptors.Определение
ChEBI: Glycine is the simplest (and the only achiral) proteinogenic amino acid, with a hydrogen atom as its side chain. It has a role as a nutraceutical, a hepatoprotective agent, an EC 2.1.2.1 (glycine hydroxymethyltransferase) inhibitor, a NMDA receptor agonist, a micronutrient, a fundamental metabolite and a neurotransmitter. It is an alpha-amino acid, a serine family amino acid and a proteinogenic amino acid. It is a conjugate base of a glycinium. It is a conjugate acid of a glycinate. It is a tautomer of a glycine zwitterion.Подготовка
From chloroacetic acid and ammonia; from protein sources, such as gelatin and silk fbroin; from ammonium bicarbonate and sodium cyanide; by catalytic cleavage of serine; from hydrobromic acid and methyleneaminoacetonitrile.Методы производства
Chemical synthesis is the most suitable method of preparation of glycine. Amination of chloroacetic acid and the hydrolysis of aminoacetonitrile are the favored methods of production.Биосинтез
Glycine is not essential to the human diet, as it is biosynthesized in the body from the amino acid serine, which is in turn derived from 3-phospho glycerate. In most organisms, the enzyme Serine hydroxy methyl transferase catalyses this transformation via the cofactor pyridoxal phosphate :serine + tetra hydro folate → glycine +N5,N10-Methylene tetrahydrofolate + H2O
In the liver of vertebrates, glycine synthesis is catalyzed by glycine synthase (also called glycine cleavage enzyme). This conversion is readily reversible : CO2 + NH4+ + N5,N10-Methylene tetra hydro folate + NADH + H+→ Glycine + tetrahydrofolate +NAD+
Glycine is coded by codons GGU, GGC, GGA and GGG. Most proteins incorporate only small quantities of glycine. A notable exception is collagen, which contains about 35 % glycine.
Биологические функции
The principal function of glycine is as a precursor to proteins. It is also a building block to numerous natural products.As a biosynthetic intermediate
In higher eukaryotes, D-Aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinyl-CoA. Glycine provides the central C2N subunit of all purines.
As a neurotransmitter
Glycine is an inhibitory neurotransmitter in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an Inhibitory postsynaptic potentia (IPSP). Strychnine is a strong antagonist at ionotropic glycine receptors, whereas bicuculline is a weak one. Glycine is a required coagonist along with glutamate for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behaviour is facilitated at the (NMDA) glutaminergic receptors which are excitatory. The LD50 of glycine is 7930 mg / kg in rats (oral), and it usually causes death by hyperexcitability. .
Общее описание
White crystals.Реакции воздуха и воды
Water soluble.Профиль реактивности
An amino acid. A 0.2M aqueous solution has a pH of 4.0., so acts as a weak acid. Has characteristics of both acid and base.Опасность
Use in fats restricted to 0.01%.Пожароопасность
LOW. Ignites at very high temperatures.Сельскохозяйственное использование
Glycine is the simplest naturally occurring amino acid and is a constituent of most proteins. Its formula is H2N·CH2·COOH.Фармацевтические приложения
Glycine is routinely used as a cofreeze-dried excipient in protein formulations owing to its ability to form a strong, porous, and elegant cake structure in the final lyophilized product. It is one of the most frequently utilized excipients in freeze-dried injectable formulations owing to its advantageous freeze-drying properties.Glycine has been investigated as a disintegration accelerant in fast-disintegrating formulations owing to its excellent wetting nature.It is also used as a buffering agent and conditioner in cosmetics.
Glycine may be used along with antacids in the treatment of gastric hyperacidity, and it may also be included in aspirin preparations to aid the reduction of gastric irritation.
Биологическая активность
One of the major inhibitory neurotransmitters in the mammalian CNS, predominantly active in the spinal cord and brain stem. Also acts as a modulator of excitatory amino acid transmission mediated by NMDA receptors. Also available as part of the NMDA Receptor - Glycine Site Tocriset™ .Профиль безопасности
Moderately toxic by intravenous route. Mildly toxic by ingestion. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx.Безопасность
Glycine is used as a sweetener, buffering agent, and dietary supplement. The pure form of glycine is moderately toxic by the IV route and mildly toxic by ingestion.Systemic absorption of glycine irrigation solutions can lead to disturbances of fluid and electrolyte balance and cardiovascular and pulmonary disorders.
LD50 (mouse, IP): 4.45 g/kg
LD50 (mouse, IV): 2.37 g/kg
LD50 (mouse, oral): 4.92 g/kg
LD50 (mouse, SC): 5.06 g/kg
LD50 (rat, IV): 2.6 g/kg
LD50 (rat, oral): 7.93 g/kg
LD50 (rat, SC): 5.2 g/kg
хранилище
Glycine starts to decompose at 233°C. Store in well-closed containers. Glycine irrigation solutions (95–105% glycine) should be stored in single dose containers, preferably type I or type II glass.Методы очистки
Crystallise glycine from distilled water by dissolving at 90-95o, filtering, cooling to about -5o, and draining the crystals centrifugally. Alternatively, crystallise it from distilled water by addition of MeOH or EtOH (e.g. 50g dissolved in 100mL of warm water, and 400mL of MeOH is added). The crystals are washed with MeOH or EtOH, then with diethyl ether. Likely impurities are ammonium glycinate, iminodiacetic acid, nitrilotriacetic acid or/and ammonium chloride. [Greenstein & Winitz The Chemistry of the Amino Acids J. Wiley, Vol 3 p 1955 1961, Beilstein 4 IV 2349.]Несовместимости
Glycine may undergo Maillard reactions with amino acids to produce yellowing or browning. Reducing sugars will also interact with secondary amines to form an imine, but without any accompanying yellow-brown discoloration.Регуляторный статус
GRAS listed. Accepted for use as a food additive in Europe. Included in the FDA Inactive Ingredients Database (IM, IV, SC injections; oral; rectal) and approved for irrigant solutions. Included in parenteral (powders for injection; solutions for injection; vaccines; kits for implant) and nonparenteral (orodispersible tablets/oral lyophilizate; powders for inhalation; powders for oral solution; tablets) formulations licensed in the UK.Глицин запасные части и сырье
сырьё
1of7
запасной предмет
- 2,2,3,3-Tetramethylcyclopropanecarboxylic кислота
- 2,2-Dimethyl-3-(2-methylpropyl)cyclopropanecarboxylic acid p-(methoxymethyl)benzyl ester
- Тиопронин
- Antistaling agent
- 4-Methyl-3-nitroanisole
- 6-Метоксииндол
- 3,4,5-триметоксибензиламин
- novel anticancer microsphere with multifunction
- 2'-IODOHIPPURIC ACID
- Альфа-ацетамидокоричная кислота
- 5-метокси-2-метиланилин
- N-Карбобензилоксиглицин
- H-TYR-GLY-GLY-PHE-MET-OH
- 3-хлорбензиламин
- DL-треонин
- 4-Methylhippuric кислота
- HYDANTOIC ACID
- 6-Methoxy-1H-indole-3-carbaldehyde
- TOSUFLOXACIN TOSILATE
- Glyphosine
- 2,4,6-трифторфенил изотиоциана
- 2-hydroxy-3-phenyl-L-alanine
- Гидрохлорид метилового эфира глицина
- 1H-ТЕТРАЗОЛ-1-АЦЕТИЛХЛОРИД
- Iprodione
1of8
Глицин поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-031166619888 +86-19333738986 |
China | 30 | 58 | ||
| +862156762820 +86-13564624040 |
China | 7707 | 58 | ||
| +8617330018573 | China | 248 | 58 | ||
| +86-15271838296; +8615271838296 |
China | 1512 | 58 | ||
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | ||
| +86-0576225566889 +86-13454675544 |
China | 12272 | 58 | ||
| +8615350851019 | China | 1001 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8615571922873 | China | 102 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 |