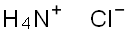
Аммония хлорид
- английское имяAmmonium chloride
- CAS №12125-02-9
- CBNumberCB7129971
- ФормулаClH4N
- мольный вес53.49
- EINECS235-186-4
- номер MDLMFCD00011420
- файл Mol12125-02-9.mol
| Температура плавления | 340 °C (subl.) (lit.) |
| Температура кипения | 100 °C750 mm Hg |
| плотность | 1.52 |
| плотность пара | 1.9 (vs air) |
| давление пара | 1 mm Hg ( 160.4 °C) |
| показатель преломления | 1.642 |
| FEMA | 4494 | AMMONIA (ALSO INCLUDES AMMONIUM CHLORIDE) |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | H2O: 1 M at 20 °C, clear, colorless |
| пка | 9.25[at 20 ℃] |
| форма | Solid |
| цвет | White |
| Удельный вес | 1.53 |
| РН | 4.7 (200g/l, H2O, 25℃)(External MSDS) |
| Запах | odorless |
| Odor Type | ammoniacal |
| Растворимость в воде | soluble |
| Чувствительный | Hygroscopic |
| λмакс | λ: 260 nm Amax: ≤0.021 λ: 280 nm Amax: ≤0.019 |
| Мерк | 14,509 |
| БРН | 4371014 |
| Диэлектрическая постоянная | 7(0.0℃) |
| Пределы воздействия | ACGIH: TWA 10 mg/m3; STEL 20 mg/m3 NIOSH: TWA 10 mg/m3; STEL 20 mg/m3 |
| Стабильность | Stable. Incompatible with strong acids, strong bases. |
| Справочник по базе данных CAS | 12125-02-9(CAS DataBase Reference) |
| FDA 21 CFR | 184.1138; 310.545 |
| Вещества, добавляемые в пищу (ранее EAFUS) | AMMONIUM CHLORIDE |
| Специальный комитет по веществам GRAS | Ammonium chloride |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | 01Q9PC255D |
| Код УВД | B05XA04,G04BA01 |
| Справочник по химии NIST | Ammonium chloride(12125-02-9) |
| Система регистрации веществ EPA | Ammonium chloride (12125-02-9) |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-36-41-37/38 | |||||||||
| Заявления о безопасности | 22-36-26 | |||||||||
| РИДАДР | UN 3077 9 / PGIII | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 10 mg/m3, STEL: 20 mg/m3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | BP4550000 | |||||||||
| Температура самовоспламенения | >400 °C | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 9 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28271000 | |||||||||
| Банк данных об опасных веществах | 12125-02-9(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in rats (mg/kg): 30 i.m. (Boyd, Seymour); LD50 in rats (mg/kg): 1650 orally (Smeets) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
Аммония хлорид химические свойства, назначение, производство
Химические свойства
Ammonium chloride,Nl4CI, also known as ammoniae, salmiai,and ammonium nituriate,is a white crystalline solid. It is soluble in water, aqueous solutionsof ammonia, and is slightly soluble in methyl alcohol. Ammonium chloride is found in natureas a sublimation productof volcanic activity, or is produced by neutralizing HCI(either in liquid or gaseousphase) with NH3 gas or liquid NH40H then evaporating the excess H20. The salt decomposes at350°C and sublimes under controlled conditions at 520 °C. Ammoniumchlorideis used as an electrolyte in dry cell batteries,as a fluxfor soldering, tinningandgalvanizing, andas a processing ingredientin textile printing and hide tanning. Use as a source of nitrogen for fertilizersis limited because of the possible build up of damaging chloride residuals in the soil.Физические свойства
Colorless cubic crystals or white granular powder; saline taste; odorless; hygroscopic; does not melt but sublimes on heating at 340°C; vapor pressure 48.75 torr at 250°C and 251.2 torr at 300°C; density 1.5274 g/cm3 at 25°C; refractive index 1.642; readily dissolves in water, solubility: 229 g and 271 g/L solution at O°C and 20°C, respectively; solubility lowered by alkali metal chlorides and HCl; dissolution lowers the temperature of the solution; sparingly soluble in alcohols (6 g/L at 19°C) and soluble in liquid NH3; insoluble in acetone and ether.Вхождение
Ammonium chloride occurs in nature in crevices near volcanoes. Also, it is found in smoke when burning dry camel or donkey dung as fuel. Important applications of this compound include the manufacture of dry cells for batteries; as a metal cleaner in soldering; as a flux in tin coating and galvanizing; in fertilizers; in pharmaceutical applications as a diuretic, or diaphoretic expectorant; and as an analytical standard in ammonia analysis. Also, it is used in freezing mixtures; washing powders; lustering cotton; in safety explosives and in dyeing and tanning.прикладной
Ammonium chloride is also known as sal ammoniac. white crystals made by ammonia salts acting upon hydrochloric acid followed by crystallization. It was used as a halide in many processes, including the salted paper, albumen paper, albumen opaltype, and gelatin emulsion processes. ammonium chloride is also used as a thickener and as an additive in non-alcoholic toners. According to cosmetic formulators, the ammonium component provides the tingling or stinging sensation that some people associate with toners or aftershaves, and which, in regular toners, is usually provided by the alcohol content. Ammonium chloride’s use is the result of preference in formulation feel.Подготовка
Ammonium chloride is prepared commercially by reacting ammonia with hydrochloric acid. It may be prepared by fractional crystallization from a solution containing ammonium sulphate and sodium chloride or ammonium carbonate and calcium chloride. Because of it sease of preparation it can be manufacture dindustrially along side any plant that uses or produces ammonia. Ammonium chloride is used in dry cells, metal finishing, and in the preparation of cotton for dyeing and printing.Определение
ChEBI: Ammonium chloride is an inorganic chloride having ammonium as the counterion. It has a role as a ferroptosis inhibitor. It is an inorganic chloride and an ammonium salt.Общее описание
Ammonium chloride is a white crystalline solid. Ammonium chloride is soluble in water(37%). The primary hazard is the threat posed to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium chloride is used to make other ammonium compounds, as a soldering flux, as a fertilizer, and for many other uses.Реакции воздуха и воды
Soluble in water. Slowly releases hydrogen chloride [USCG, 1999].Профиль реактивности
Acidic salts, such as Ammonium chloride , are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.Опасность
Eye and upper respiratory tract irritant.Угроза здоровью
Inhalation of fumes irritates respiratory passages. Ingestion irritates mouth and stomach. Fumes are irritating to eyes. Contact with skin may cause irritation.Безопасность
Ammonium chloride is used in oral pharmaceutical formulations. The pure form of ammonium chloride is toxic by SC, IV, and IM routes, and moderately toxic by other routes. Potential symptoms of overexposure to fumes are irritation of eyes, skin, respiratory system: cough, dyspnea, and pulmonary sensitization. Ammonium salts are an irritant to the gastric mucosa and may induce nausea and vomiting.LD50 (mouse, IP): 1.44 g/kg
LD50 (mouse, oral): 1.3 g/kg
LD50 (rat, IM): 0.03 g/kg
LD50 (rat, oral): 1.65 g/kg
Возможный контакт
Ammonium chloride is used as an industrial chemical, pharmaceutical, and veterinary drug; to make dry batteries; in galvanizing; as a soldering flux.хранилище
Ammonium chloride is chemically stable. It decomposes completely at 3388℃ to form ammonia and hydrochloric acid. Store in airtight containers in a cool, dry place.Перевозки
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.Методы очистки
Crystallise it several times from conductivity water (1.5mL/g) between 90o and 0o. It sublimes. After one crystallisation, ACS grade has: metal(ppm) As (1.2), K (1), Sb (7.2), V (10.2). [Becher in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 812 1963.]Несовместимости
Ammonium chloride is incompatible with strong acids and strong bases. It reacts violently with ammonium nitrate and potassium chlorate, causing fire and explosion hazards. It also attacks copper and its compounds.Утилизация отходов
Pretreatment involves addition of sodium hydroxide to liberate ammonia and form the soluble sodium salt. The liberated ammonia can be recovered and sold. After dilution to the permitted provisional limit, the sodium salt can be discharged into a stream or sewer.Регуляторный статус
GRAS listed. Included in the FDA Inactive Ingredients Database (oral syrup, tablets). Accepted for use as a food additive in Europe. Included in medicines licensed in the UK (eye drops; oral syrup).Аммония хлорид запасные части и сырье
сырьё
1of4
запасной предмет
- 3,4-Дихлор-1 ,2,5-тиадиазол
- THIOTHIAMINE
- 2-фуральдегид диэтилацеталь
- 1-PHENYL-3-THIOSEMICARBAZIDE
- 4-(4-хлорфенил)-1,2,3,6-тетрагидропиридин гидрохлорид
- 2-АМИНО-4,6-ДИМЕТИЛ-3-ПИРИДИНКАРБОКСАМИД
- эластазы
- 4-Methylbenzene-1-carboximidamide hydrochloride
- анамицина сульфа
- Dye-fixing agent M
- Amino tris(methylene phosphonic acid)
- 3-PYRIDINECARBOXAMIDINE
- Этил бензоилформиа
- 4-METHOXY-BENZAMIDINE
- Methyl 3-aminosulfonylthiophene-2-carboxylate
- CYCLOLEUCINOL
- Praseodymium-Zircon Yellow
- 2-Хлор-5-(хлорметил) тиофена
- TRIHEXYLPHOSPHINE
- xylene isomerization catalysts
- new type low toxic urea-formalde-hyde adhesive A-01-B
- Пиридин-2-карбoксимидамид гидрохлорид
- 1-нафтил изоциана
- 4-AMIDINOPYRIDINIUM CHLORIDE
- 3-МЕТИЛТИОФЕН-2-КАРБОКСАМИД
- 4-хлорбензамидин гидрохлорид
- 4-METHYL-BENZAMIDINE
- 5-(AMINOMETHYL)-5-METHYLPYRROLIDIN-2-ONE
- 2,5-Dichlorothiophene-3-sulfonamide
- 2-нафтил изоциана
1of8
Аммония хлорид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8613607293350 | China | 1 | 58 | ||
| +8613573296305 | China | 298 | 58 | ||
| +86-031166619888 +86-19333738986 |
China | 25 | 58 | ||
| +86-029-81138252 +86-18789408387 |
China | 2873 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12459 | 58 | ||
| +86-86-13583358881 +8618560316533 |
China | 3146 | 58 | ||
| +86-66697723 +86-17703311139 |
China | 563 | 58 | ||
| +86-19930503253; +8619930503252 |
China | 5838 | 58 | ||
| +8617756083858 | China | 994 | 58 | ||
| +8619931165850 | China | 1000 | 58 |