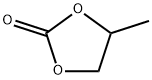
ПРОПИЛЕНКАРБОНАТ
- английское имяPropylene carbonate
- CAS №108-32-7
- CBNumberCB8852744
- ФормулаC4H6O3
- мольный вес102.09
- EINECS203-572-1
- номер MDLMFCD00005385
- файл Mol108-32-7.mol
химическое свойство
| Температура плавления | -55 °C (lit.) |
| Температура кипения | 240 °C (lit.) |
| плотность | 1.204 g/mL at 25 °C (lit.) |
| давление пара | 0.13 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 270 °F |
| температура хранения | Store below +30°C. |
| растворимость | 240g/l |
| форма | Liquid |
| пка | 3.92[at 20 ℃] |
| цвет | Clear |
| Удельный вес | 1.209 (20/4℃) |
| Относительная полярность | 6 |
| РН | 7.0 (200g/l, H2O, 20℃) |
| Запах | odorless |
| Пределы взрываемости | 1.8-14.3%(V) |
| Растворимость в воде | 240 g/L (20 ºC) |
| λмакс | λ: 235 nm Amax: 1.00 λ: 280 nm Amax: 0.50 λ: 300 nm Amax: 0.30 λ: 350 nm Amax: 0.05 λ: 375-400 nm Amax: 0.01 |
| БРН | 107913 |
| Диэлектрическая постоянная | 64.4(Ambient) |
| Стабильность | Stable. Incompatible with strong oxidizing agents, acids, bases, reducing agents. Protect from contact with moist air or water. |
| ИнЧИКей | RUOJZAUFBMNUDX-UHFFFAOYSA-N |
| LogP | -0.41 at 20℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | PROPYLENE CARBONATE |
| FDA 21 CFR | 175.105 |
| Справочник по базе данных CAS | 108-32-7(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | 8D08K3S51E |
| Справочник по химии NIST | Propylene carbonate(108-32-7) |
| Система регистрации веществ EPA | Propylene carbonate (108-32-7) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
больше
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36 | |||||||||
| Заявления о безопасности | 26-37/39 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | FF9650000 | |||||||||
| F | 3-10 | |||||||||
| Температура самовоспламенения | 851 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29209010 | |||||||||
| Банк данных об опасных веществах | 108-32-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: > 5000 mg/kg LD50 dermal Rabbit > 20000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H319:При попадании в глаза вызывает выраженное раздражение.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P337+P313:Если раздражение глаз не проходит обратиться за медицинской помощью.
ПРОПИЛЕНКАРБОНАТ химические свойства, назначение, производство
Химические свойства
Propylene carbonate is a clear, colorless, mobile liquid, with a faint odor.Использование
propylene carbonate is used in chemical reactions as a solvent, plasticizer, solubilizer, or dilutent. It is also used in the synthesis of solar cells as well as lithium ion batteries.Методы производства
Propylene carbonate may be prepared by the reaction of sodium bicarbonate with propylene chlorohydrin.Общее описание
Propylene carbonate is a cyclic carbonate that is commonly used as a solvent and as a reactive intermediate in organic synthesis. It is being considered as a potential electrochemical solvent due to its low vapor pressure, high dielectric constant and high chemical stability.Propylene carbonate can be synthesized from propylene oxide and CO2. Optically active form of propylene carbonate can be prepared from the reaction between CO2 and racemic epoxides. Decomposition of propylene carbonate on the graphite electrode in lithium batteries results in the formation of a lithium intercalated compound.
Фармацевтические приложения
Propylene carbonate is used mainly as a solvent in oral and topical pharmaceutical formulations.In topical applications, propylene carbonate has been used in combination with propylene glycol as a solvent for corticosteroids. The corticosteroid is dissolved in the solvent mixture to yield microdroplets that can then be dispersed in petrolatum.Propylene carbonate has been used as a dispensing solvent in topical preparations.
Propylene carbonate has also been used in hard gelatin capsules as a nonvolatile, stabilizing, liquid carrier. For formulations with a low dosage of active drug, a uniform drug content may be obtained by dissolving the drug in propylene carbonate and then spraying this solution on to a solid carrier such as compressible sugar; the sugar may then be filled into hard gelatin capsules
Propylene carbonate may additionally be used as a solvent, at room and elevated temperatures, for many cellulose-based polymers and plasticizers. Propylene carbonate is also used in cosmetics.
Безопасность
Propylene Carbonate is listed in The Design for the Environment (DfE) Safer Chemistry Program by the EPA as a Solvent category and indicated by the Green circle, meaning the chemical has been verified to be of low concern for human and environmental health based on experimental and modeled data.In animal studies, propylene carbonate was found to cause tissue necrosis after parenteral administration.
(mouse, oral): 20.7 g/kg
(mouse, SC): 15.8 g/kg
(rat, oral): 29 g/kg
(rat, SC): 11.1 g/kg
хранилище
Propylene carbonate and its aqueous solutions are stable but may degrade in the presence of acids or bases, or upon heating;Store in a well-closed container in a cool, dry place.
Методы очистки
It is manufactured by reaction of 1,2-propylene oxide with CO2 in the presence of a catalyst (quaternary ammonium halide). Contaminants include propylene oxide, carbon dioxide, 1,2-and 1,3-propanediols, allyl alcohol and ethylene carbonate. It can be purified by percolation through molecular sieves (Linde 5A, dried at 350o for 14hours under a stream of argon), followed by distillation under a vacuum. [Jasinski & Kirkland Anal Chem 39 163 1967.] It can be stored over molecular sieves under an inert gas atmosphere. When purified in this way it contains less than 2ppm of water. Activated alumina and dried CaO have also been used as drying agents prior to fractional distillation under reduced pressure. It has been dried with 3A molecular sieves and distilled under nitrogen in the presence of p-toluenesulfonic acid, then redistilled and the middle fraction collected. [Beilstein 19 III/IV 1564, 19/4 V 21.]Несовместимости
Propylene carbonate hydrolyzes rapidly in the presence of strong acids and bases, forming mainly propylene oxide and carbon dioxide. Propylene carbonate can also react with primary and secondary amines to yield carbamates.Регуляторный статус
Included in the FDA Inactive Ingredients Database (topical ointments). Included in the Canadian List of Acceptable Nonmedicinal Ingredients.ПРОПИЛЕНКАРБОНАТ запасные части и сырье
сырьё
1of2
запасной предмет
1of2
ПРОПИЛЕНКАРБОНАТ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 571-88938639 +8617705817739 |
China | 52849 | 58 | ||
| +8617653113219 | China | 2737 | 58 | ||
| 15957180504 | China | 193 | 58 | ||
| +86-025-83346564; +8613182976929 |
China | 122 | 58 | ||
| +86-0576225566889 +86-13454675544 |
China | 12272 | 58 | ||
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617756083858 | China | 973 | 58 |