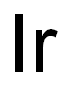
Иридий
- английское имяIridium
- CAS №7439-88-5
- CBNumberCB8780841
- ФормулаIr
- мольный вес192.22
- EINECS231-095-9
- номер MDLMFCD00011062
- файл Mol7439-88-5.mol
| Температура плавления | 2450 °C (lit.) |
| Температура кипения | 4130 °C (lit.) |
| плотность | 22.65 g/cm3 (lit.) |
| температура хранения | Flammables area |
| растворимость | Soluble in aqua regia. |
| форма | wire |
| цвет | White to pale yellow |
| Удельный вес | 22.421 |
| удельное сопротивление | 4.71 μΩ-cm |
| Растворимость в воде | INSOLUBLE |
| Мерк | 13,5102 |
| Пределы воздействия | ACGIH: Ceiling 2 ppm OSHA: Ceiling 5 ppm(7 mg/m3) NIOSH: IDLH 50 ppm; Ceiling 5 ppm(7 mg/m3) |
| Справочник по базе данных CAS | 7439-88-5(CAS DataBase Reference) |
| FDA UNII | 44448S9773 |
| Справочник по химии NIST | Iridium(7439-88-5) |
| Система регистрации веществ EPA | Iridium (7439-88-5) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | F,Xi | |||||||||
| Заявления о рисках | 36-36/37/38-11 | |||||||||
| Заявления о безопасности | 16-26-36/37/39 | |||||||||
| РИДАДР | UN 3089 4.1/PG 2 | |||||||||
| WGK Германия | 3 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 4.1 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28439000 | |||||||||
| Банк данных об опасных веществах | 7439-88-5(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
вредная бумага
H228:Воспламеняющееся твердое вещество.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P240:Заземлить и электрически соединить контейнер и приемное оборудование.
P241:Использовать взрывобезопасное оборудование и освещение.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P370+P378:При пожаре тушить сухим песком, сухим химическим порошком или спиртостойкой пеной.
Иридий химические свойства, назначение, производство
Химические свойства
Black PowderФизические свойства
Iridium is a hard, brittle, white, metallic substance that is almost impossible to machine.It is neither ductile nor malleable. Iridium will only oxidize at high temperatures and is themost corrosive-resistant metal known. This is why it was used to make the standard meter barthat is an alloy of 90% platinum and 10% iridium.At about the time of the French Revolution, it was decided to determine the length ofthe meter bar by first calculating the distance from the North Pole to the equator runningthrough Paris. This distance was then divided into equal lengths of 1/10,000,000. A singleunit of this distance was then called a “meter” (“measure” in Greek). This platinum-iridiummeter bar, currently preserved in France, was for many years the standard unit of lengthin the metric system that is based on the decimal system. However, this metal bar is nolonger used as the standard meter. Instead, the meter is now defined by scientists in termsof the length of the path traveled by light in a vacuum at the time of 1/299,792,458 of asecond.
Iridium is highly resistant to attack by other chemicals and is one of the most dense elementsfound on Earth. Its melting point is 2,410°C, its boiling point is 4,130°C, and itsdensity is 22.560 g/cm3.
Изотопы
There are 55 isotopes of iridium, two of which are stable and account for theelement’s total existence on Earth. Those two are Ir-191, which makes up 37.3% of theamount in the Earth’s crust, and Ir-193, which constitutes 62.7% of iridium’s existenceon Earth. All the other 53 isotopes of iridium are radioactive with half-lives ranging froma few microseconds to a few hours or days and up to a few hundred years. Theseunstable isotopes are all artificially produced.Происхождение имени
The name iridium comes from the Latin word iris, meaning “rainbow,” because of the element’s highly colored salts.Вхождение
Iridium is the 83rd most abundant element and is found mixed with platinum, osmium,and nickel ores. The minerals containing iridium are found in Russia, South Africa, Canada,and Alaska.Iridium metal is separated from its other metal ores when the combined minerals aredissolved with a strong acid know as aqua regia, which is a mixture of 25% nitric acid and75% hydrochloric acid. Aqua regia is the only acid that will dissolve platinum and gold.Once the platinum and other metals are dissolved, the iridium, which is insoluble in thisstrong acid, becomes the residue. The refined iridium ends up in the form of either powderor crystals.
An interesting story as to how most of the iridium appeared on Earth was explainedrecently by scientists who discovered a thin layer of iridium in the sediments that were laiddown in the Earth’s crust at the end of the Cretaceous period. This was a period about 65 millionyears ago when meteors and asteroids crashed into the Earth. These extraterrestrial bodiescontained a high percentage of iridium. Dust from the impact spread around the Earth andblocked the sun for months, resulting in the extinction of many plants and animals, includingthe dinosaurs. This extensive dust cloud also deposited a thin coat of the element iridium thatwas contained in the fiery bolides.
Характеристики
Iridium is one of the so-called platinum group of 6 transition elements (Ru, Rh, and Pd ofperiod 5 and Os, Ir, and Pt of period 6). It is resistant to strong acids, including aqua regia.It is the only metal that can be used in equipment that must withstand temperatures up to2,300°C or 4,170°F. Iridium can be poured into casts after it becomes molten. As it cools, itbecomes crystalline and, while in this state, can be pulled into wires and formed into sheets.Unlike steel, which becomes more malleable (less brittle) after annealing (a process of heatingfollowed by slowly cooling), iridium is just the opposite—it becomes more brittle and impossibleto work into shapes after cooling.Использование
Iridium’s most common use is as an alloy metal that, when added to platinum, makes itharder and more durable. It is also mixed with other metals to make electrical contacts, thermocouples(two dissimilar metals joined to form a special type of thermometer), and instrumentsthat will withstand high temperatures without breaking down. It is also used to makespecial laboratory vessels because iridium will not react with most chemical substances. Analloy of iridium and platinum is used as the standard kilogram weight because it is noncorrosiveand will not oxidize and, thus, change its weight over long periods of time.The radioisotope, iridium-192, is used to treat cancer and to take X-ray pictures of metalcastings to detect flaws. Iridium is also used as a catalyst for several chemical reactions.
Определение
iridium: Symbol Ir. A silvery metallictransition element; a.n. 77; r.a.m. 192.20;r.d. 22.42; m.p. 2410°C; b.p. 4130°C.It occurs with platinum and ismainly used in alloys with platinumand osmium. The element forms arange of iridium(III) and iridium(IV)complexes. It was discovered in 1804by Smithson Tennant (1761–1815).Опасность
The elemental metal form of iridium is almost completely inert and does not oxidizeat room temperatures. But, as with several of the other metals in the platinum group,several of iridium’s compounds are toxic. The dust and powder should not be inhaled oringested.Промышленное использование
Iridium (symbol Ir) is a grayish-white metal ofextreme hardness. It is insoluble in all acids andin aqua regia. The melting point is 2447°C, andthe specific gravity is 22.50. It occurs naturallywith the metal osmium as an alloy, known asosmiridium, 30 to 60% osmium, used chieflyfor making fountain-pen points and instrumentpivots.Iridium is employed as a hardener for platinum,the jewelry alloys usually containing10%. With 35% iridium the tensile strength ofplatinum is increased to 965 MPa. Iridium wireis used in spark plugs because it resists attackof leaded aviation fuels.
Because of its scarcity and high cost, applications of iridium are severely limited. Although iridium metal and many of its complex compounds are good catalysts, no large-scale commercial application for these has been developed. In general, other platinum metals have superior catalytic properties. Platinum-iridium alloys are used for electrodes in spark plugs that are unusually resistant to fouling by antiknock lead additives. Iridium rhodium thermocouples are used for high-temperature applications, where they have unique stability.
Методы очистки
Iridium is a silver white hard solid which oxidises on the surface in air. Scrape the outer tarnished layer until silver clear and store it under paraffin. It is stable to acids but dissolves in aqua regia. [Gilchrist Chem Rev 32 277 1943.]Структура и конформация
The space lattice of Ir belongs to the cubic system, and its face-centered cubic lattice has a lattice constant of a=0.38312 nm and Ir–Ir=0.2709 nm (18℃).Иридий запасные части и сырье
сырьё
запасной предмет
Иридий поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +undefined-86-1523780-4566 +undefined15237804566 |
China | 511 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| 323-480-4688 | United States | 989 | 55 | ||
| 18871490254 | CHINA | 28172 | 58 | ||
| +8619521488211 | China | 2558 | 58 | ||
| +86-0371-86658258 +8613203830695 |
China | 29809 | 58 | ||
| 18503026267 | CHINA | 9636 | 58 | ||
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 |