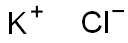
Калия хлорид
- английское имяPotassium chloride
- CAS №7447-40-7
- CBNumberCB9137176
- ФормулаKCl
- мольный вес74.55
- EINECS231-211-8
- номер MDLMFCD00011360
- файл Mol7447-40-7.mol
химическое свойство
| Температура плавления | 770 °C (lit.) | ||||||||||||||
| Температура кипения | 1420°C | ||||||||||||||
| плотность | 1.98 g/mL at 25 °C (lit.) | ||||||||||||||
| Плотность накопления | 1000kg/m3 | ||||||||||||||
| показатель преломления | n |
||||||||||||||
| Fp | 1500°C | ||||||||||||||
| температура хранения | 2-8°C | ||||||||||||||
| растворимость | H2O: soluble | ||||||||||||||
| форма | random crystals | ||||||||||||||
| Удельный вес | 1.984 | ||||||||||||||
| цвет | White | ||||||||||||||
| РН | 5.5-8.0 (20℃, 50mg/mL in H2O) | ||||||||||||||
| Запах | Odorless | ||||||||||||||
| Водородный показатель | 7 | ||||||||||||||
| Flame Color | Light Purple | ||||||||||||||
| Биологические источники | rabbit | ||||||||||||||
| Растворимость в воде | 340 g/L (20 ºC) | ||||||||||||||
| Чувствительный | Hygroscopic | ||||||||||||||
| λмакс | λ: 260 nm Amax: 0.02 λ: 280 nm Amax: 0.01 |
||||||||||||||
| Кристальная структура | NaCl type | ||||||||||||||
| crystal system | Cube | ||||||||||||||
| Мерк | 14,7621 | ||||||||||||||
| Сублимация | 1500 ºC | ||||||||||||||
| БРН | 1711999 | ||||||||||||||
| Space group | Fm3m | ||||||||||||||
| Lattice constant |
|
||||||||||||||
| Диэлектрическая постоянная | 4.6(Ambient) | ||||||||||||||
| BCS Class | 1 | ||||||||||||||
| Стабильность | Stable. Incompatible with strong oxidizing agents, strong acids. Protect from moisture. Hygroscopic. | ||||||||||||||
| ИнЧИКей | WCUXLLCKKVVCTQ-UHFFFAOYSA-M | ||||||||||||||
| Справочник по базе данных CAS | 7447-40-7(CAS DataBase Reference) | ||||||||||||||
| FDA 21 CFR | 184.1622; 582.5622; 558.128; 558.311 | ||||||||||||||
| Вещества, добавляемые в пищу (ранее EAFUS) | POTASSIUM CHLORIDE | ||||||||||||||
| Специальный комитет по веществам GRAS | Potassium chloride | ||||||||||||||
| Рейтинг продуктов питания EWG | 1 | ||||||||||||||
| FDA UNII | 660YQ98I10 | ||||||||||||||
| Код УВД | A12BA01,A12BA51,B05XA01 | ||||||||||||||
| Справочник по химии NIST | Potassium chloride(7447-40-7) | ||||||||||||||
| Система регистрации веществ EPA | Potassium chloride (7447-40-7) | ||||||||||||||
| UNSPSC Code | 41116107 | ||||||||||||||
| NACRES | ND.02 |
больше
| Коды опасности | Xi,C,F,Xn,T | |||||||||
| Заявления о рисках | 36-34-11-36/37/38-40-61-60 | |||||||||
| Заявления о безопасности | 24/25-39-26-22-23-45-36/37/39-16-36/37-53 | |||||||||
| РИДАДР | UN 1824 8 / PGII | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | TS8050000 | |||||||||
| F | 8 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 31042090 | |||||||||
| Банк данных об опасных веществах | 7447-40-7(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in Rabbit: 2600 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
опасность
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
Калия хлорид химические свойства, назначение, производство
Описание
Potassium chloride (KCl) is a metal halide salt that is used in a variety of areas. The dominant application of potassium chloride is to serve as a fertilizer, which offers potassium to plants and prevents them from certain diseases. Besides, it can be applied in food and medical industry. As a treatment for hypokalemia, potassium chloride pills are taken to balance the blood's potassium levels and prevent potassium deficiency in the blood. In food industry, it serves as a electrolyte replenisher and a good salt substitute for food, as well as a firming agent to give consistent texture to food, thus to strengthen its structure.Химические свойства
Potassium chloride occurs as odorless, colorless crystals or a white crystalline powder, with an unpleasant, saline taste. The crystal lattice is a face-centered cubic structure. Potassium chloride occurs naturally as the mineralsylvite (KCl) and as carnallite(KCl·MgCl2·6H2O); it is produced industriallyby fractional crystallizationof these deposits or of solutions fromlake brines. It has the interesting property of being more soluble than sodium chloride in hot water but less soluble in cold. It has low toxicity.Использование
- Potassium chloride is a widely used reagent in biochemistry and molecular biology. It is a component of phosphate buffered saline (PBS, Product No. P 3813) and of polymerase chain reaction (PCR) buffer (50 mM KCl).
- KCl is also used in studies of ion transport and potassium channels.
- KCl is also utilized in the solubilization, extraction, purification, and crystallization of proteins.
- The use of KCl in the crystallization of histone core octamers has been reported.
Определение
ChEBI: Potassium chloride is a metal chloride salt with a K(+) counterion. It has a role as a fertilizer. It is a potassium salt, an inorganic chloride and an inorganic potassium salt.Методы производства
Potassium chloride occurs naturally as the mineral sylvite or sylvine; it also occurs in other minerals such as sylvinite, carnallite, and kainite. Commercially, potassium chloride is obtained by the solar evaporation of brine or by the mining of mineral deposits.Всемирная организация здравоохранения(ВОЗ)
Potassium chloride has been used for many years to correct potassium deficiency. The use of fast-acting tablets has been associated with lesions of the gastro-intestinal mucosa, which have led to their general withdrawal.Общее описание
Potassium chloride (KCl) is a water-soluble metal salt that comprises of potassium and chlorine. It can be extracted from minerals and salt water. KCl can be used in industries such as cosmetics, food, biomedical, chemical and fertilizer.Реакции воздуха и воды
Hygroscopic. Water soluble.Профиль реактивности
Potassium chloride is not in general strongly reactive. Violent reaction with BrF3 and with a mixture of sulfuric acid potassium permanganate mixture . Reacts with concentrated sulfuric acid to generate fumes of hydrogen chloride.Угроза здоровью
Potassium chloride is an essential constituent of the body for intracellular osmotic pressure and buffering, cell permeability, acid-base balance, muscle contraction and nerve function.SYMPTOMS: Large doses of Potassium chloride usually induce vomiting, so acute intoxication by mouth is rare. If no pre-existing kidney damage, it is rapidly excreted. Poisoning disturbs the rhythm of heart. Large doses by mouth can cause gastrointestinal irritation, purging, weakness, and circulatory disturbances.
Пожароопасность
Flammability data is not available, but Potassium chloride is probably nonflammable.Сельскохозяйственное использование
Muriate of potash or potassium chloride (KCl), is a major potash fertilizer. It is water soluble and is generally blended with other components to make it a multi-nutrient fertilizer. It has a higher salt index than potassium sulphate and is recommended for most crops except tobacco, potato and grapes, which are sensitive to chloride ions.Фармацевтические приложения
Potassium chloride is widely used in a variety of parenteral and nonparenteral pharmaceutical formulations. Its primary use, in parenteral and ophthalmic preparations, is to produce isotonic solutions.Potassium chloride is also used therapeutically in the treatment of hypokalemia.
Many solid-dosage forms of potassium chloride exist including: tablets prepared by direct compression and granulation; effervescent tablets; coated, sustained-release tablets; sustained- release wax matrix tablets;microcapsules;pellets; and osmotic pump formulations.
Experimentally, potassium chloride is frequently used as a model drug in the development of new solid-dosage forms, particularly for sustained-release or modified-release products. Potassium chloride is also used widely in the food industry as a dietary supplement, pH control agent, stabilizer, thickener, and gelling agent. It can also be used in infant formulations.
Промышленное использование
Potassium chloride is a colorless or white crystallinecompound of the composition KCl, usedfor molten salt baths for the heat treatment ofsteels. The specific gravity is 1.987. A bathcomposed of three parts potassium chloride andtwo parts barium chloride is used for hardeningcarbon-steel drills and other tools. Steel toolsheated in this bath and quenched in a 3% sulfuricacid solution have a very bright surface.A common bath is made up of potassium chlorideand common salt and can be used for temperaturesup to 900°C.Potassium chloride is used in the porcelainenamel industry as a setting-up agent in titaniumcover coats. In general, the quantities ofpotassium chloride, when used as an electrolyte,will be approximately the same as sodiumnitrite, which it replaces. However, KCl doesnot aid tearing resistance as does nitrite. Themain advantage in using potassium chloride isthe freedom from yellowing or creaming whenused in a blue-white enamel. Potassium chloridemay exert an adverse effect on the glossand may cause a slight decrease in the acidresistingproperties of the enamel, although thelatter effect is somewhat debatable.
Профиль безопасности
A human poison by ingestion. Poison experimentally by ingestion, intravenous, and intraperitoneal routes. Human systemic effects by ingestion: nausea, blood clotting changes, carhac arrhythmias. An eye irritant. Mutation data reported. Explosive reaction with BrF3; sulfuric acid + potassium permanganate. When heated to decomposition it emits toxic fumes of K2O and Cl-.хранилище
Potassium chloride tablets become increasingly hard on storage at low humidities. However, tablets stored at 76% relative humidity showed no increase or only a slight increase in hardness.The addition of lubricants, such as 2% w/w magnesium stearate, reduces tablet hardness and hardness on aging.Aqueous potassium chloride solutions may be sterilized by autoclaving or by filtration.Potassium chloride is stable and should be stored in a well-closed container in a cool, dry place.
Методы очистки
Dissolve it in conductivity water, filter it, and saturate it with chlorine (generated from conc HCl and KMnO4). Excess chlorine is boiled off, and the KCl is precipitated by HCl (generated by dropping conc HCl into conc H2SO4). The precipitate is washed with water, dissolved in conductivity water at 90-95o, and crystallised by cooling to about -5o. The crystals are drained at the centrifuge, dried in a vacuum desiccator at room temperature, then fused in a platinum dish under N2, cooled and stored in a desiccator. Potassium chloride has also been sublimed in a stream of pre-purified N2 gas and collected by electrostatic discharge [Craig & McIntosh Can J Chem 30 448 1952].Несовместимости
Potassium chloride reacts violently with bromine trifluoride and with a mixture of sulfuric acid and potassium permanganate. The presence of hydrochloric acid, sodium chloride, and magnesium chloride decreases the solubility of potassium chloride in water. Aqueous solutions of potassium chloride form precipitates with lead and silver salts.Intravenous aqueous potassium chloride solutions are incompatible with protein hydrolysate.
Регуляторный статус
GRAS listed. Accepted as a food additive in Europe. Included in the FDA Inactive Ingredients Database (injections, ophthalmic preparations, oral capsules, and tablets). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.использованная литература
https://en.wikipedia.org/wiki/Potassium_chloridehttps://www.drugbank.ca/drugs/DB00761
http://study.com/academy/lesson/what-is-potassium-chloride-uses-formula-side-effects.html
Калия хлорид запасные части и сырье
сырьё
1of4
запасной предмет
- Compound fertilizer
- Альгиновая кислота натриевая сол
- Натрия сульфат декагидра
- Аденин
- 4-аминофенил эфир
- Nitrogen phosphorus potassium mixed fertilizer
- Ампициллин
- ^ Б-Никотинамидадениндинуклеотид
- Ртути йодид
- carnallite
- Nitrogen-Phosphorus-Potassium compound fertilizer with sulphur
- Калий тетрафтороборат
- Калия гидрокарбонат
- Tetrapotassium hexacyanoferrate trihydrate
- Silicic acid, aluminum potassium sodium salt
- Гестоден
- Mixed and compound fertilizer
- РЕАКТИВНЫЙ ФИОЛЕТОВЫЙ 5
- PROCESSEDEUCHEUMASEAWEED
- Бромат калия
- ГЕКСАФТОРСИЛИКАТ КАЛИЯ
- КАЛИЙ двухромовокислый
- Potassium dihydrogen orthophosphate
- Калия перхлорат
- Фурцелларан
- Калия хлорат
- Compound fertilizer of potassium sulfate
- Калия ферроцианид тригидрат
- Хром (III) оксид
- Potassium hexafluorozirconate
1of8
Калия хлорид поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8615531157085 | China | 8804 | 58 | |
| +86-13131129325 | China | 5887 | 58 | |
| +8617798174412 | China | 2097 | 58 | |
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | |
| +8619931165850 | China | 1000 | 58 | |
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | |
| +8618092446649 | China | 1143 | 58 | |
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | |
| +8613343047651 | China | 3692 | 58 | |
| +86-18633929156 +86-18633929156 |
China | 972 | 58 |