химическое свойство
| Температура плавления | 99 °C |
| плотность | 1.0 g/cm3(Temp: 25 °C) |
| FEMA | 2014 | ALGIN (LAMINARIA SPP. AND OTHER KELPS) |
| температура хранения | 2-8°C |
| растворимость | Slowly soluble in water forming a viscous, colloidal solution, practically insoluble in ethanol (96 per cent). |
| форма | powder |
| цвет | White to Off-white |
| Запах | odorless |
| РН | 6.0-8.0 (10mg/mL in H2O) |
| Биологические источники | synthetic |
| Вязкость | 4-12cP1% in H2O(25°C)(lit.) |
| Растворимость в воде | Soluble in water. Insoluble in alcohol, chloroform and ether. |
| Чувствительный | Hygroscopic |
| Мерк | 14,241 |
| Стабильность | Stable. Incompatible with strong acids, strong bases, strong oxidizing agents. |
| LogP | -2.88 |
| FDA 21 CFR | 184.1724; 582.7724; 173.310 |
| Вещества, добавляемые в пищу (ранее EAFUS) | ALGINATE, SODIUM |
| Специальный комитет по веществам GRAS | Sodium alginate |
| Справочник по базе данных CAS | 9005-38-3(CAS DataBase Reference) |
| FDA UNII | C269C4G2ZQ |
| Система регистрации веществ EPA | Sodium alginate (9005-38-3) |
| UNSPSC Code | 41116107 |
| NACRES | NA.72 |
больше
| Коды опасности | Xi | |||||||||
| Заявления о рисках | 36/37/38 | |||||||||
| Заявления о безопасности | 24/25-36-26 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | AZ5820000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 39131000 | |||||||||
| Банк данных об опасных веществах | 9005-38-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 oral in rat: > 5gm/kg | |||||||||
| NFPA 704: |
|
Альгиновая кислота натриевая сол химические свойства, назначение, производство
Описание
Sodium alginate is the sodium form of alginate. Alginate is a linear, anionic polysaccharide consisting of two form of 1, 4-linked hexuronic acid residues, β-d-mannuronopyranosyl (M) and α-l- guluronopyranosyl (G) residues. It can be arranged in the form of blocks of repeating M residues (MM blocks), blocks of repeating G residues (GG blocks), and blocks of mixed M and G residues (MG blocks). Commercially available alginate currently originates from algae. Alginate has wide applications. For example, one of its most important role is being used as wound dressing materials for the treatment of acute or chronic wounds. The use of alginate crosslinking to make hydrogels for cell encapsulation is also quite valuable. The emergence of various kinds of its derivatives recently has further extended its application.Химические свойства
Colorless or slightly yellow solid occurring in filamentous, granular, and powdered forms. Forms a viscous colloidal solution with water; insoluble in alcohol, ether, and chloroform. Combustible. Sodium alginate is mixed with a solution or suspension of the biocatalysts and then dropped into a calcium chloride solution to form water-insoluble calcium alginate gels that immobilize enzymes, cellular organelles, or microbial cells.Физические свойства
Sodium alginate occurs as an odorless and tasteless, white to pale yellowish-brown colored powder. insoluble in alcohol, ether or chloroform, etc. The aqueous solution of sodium alginate is stable at pH 4 to 10, and precipitates when pH < 3; hydrolysis occurs when pH > 10, and viscosity is lost at the same time.Характеристики
Sodium alginate [9005-38-3] is extracted from seaweed and is a linear copolymer of β-dmannuronic acid and α-l-guluronic acid linked by 1,4-glycosidic bonds. It forms a gel in the presence of multivalent ions, usually calcium or aluminum. The controlled entrapment of cells is simple and generally nontoxic. Various cell types can be immobilized with negligible loss of viability. However, the matrix can be solubilized in the presence of Ca2+-chelating agents such as phosphate, citrate, or ethylenediaminetetraacetic acid. The alginate matrix is mechanically weak so that the growing cells (especially plant cells) can be released from, or even disintegrate, the beads. Another drawback of the alginate method for viable animal cells is the difficulty of producing sufficiently small beads to overcome oxygen limitation in their interior.История
Sodium alginate is a natural polysaccharide product that was first described in a patent application by the British chemist Edward C C Stanford in 1881. To this day brown algae are still the main source used to extract sodium alginate from. This group includes many of the seaweeds, like kelps, found in chilly northern seas. In addition to the food industry, the gelling properties of sodium alginate have been used in medical, dental and cosmetic applications for years.Использование
- Sodium alginate can be used as a flavorless gum. It is used by the foods industry to increase viscosity and as an emulsifier. It is also used in indigestion tablets and the preparation of dental impressions.
- Sodium alginate (NaAlg) and its modified forms have been widely used as membranes in pervaporation (PV) separation of aqueous‐organic solutions because of the hydrophilic nature and versatility to modify/tune their structures to achieve the desired separation.
- Sodium alginate is a polymer which can be extracted from brown seaweed and kelps. It is one of the structural polymers that help to build the cell walls of these plants. It has some unusual properties and a wide variety of uses.
The polymer can be represented like this:
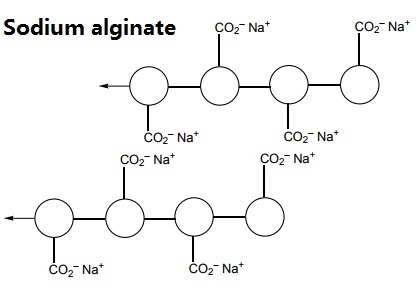
When sodium alginate is put into a solution of calcium ions, the calcium ions replace the sodium ions in the polymer. Each calcium ion can attach to two of the polymer strands. This is called cross-linking and can be represented like this:
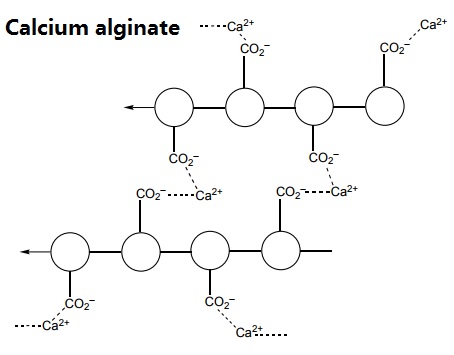
Методы производства
Alginic acid is extracted from brown seaweed and is neutralized with sodium bicarbonate to form sodium alginate.прикладной
Sodium Alginate is a gum obtained as a sodium salt of alginic acid, which is obtained from seaweed. it is coldand hot-water soluble, producing a range of viscosities. it forms irreversible gels with cal- cium salts or acids. it functions as a thickener, binder, and gelling agent in dessert gels, puddings, sauces, toppings, and edible films. In the manufacture of ice cream where it serves as a stabilizing colloid, insuring creamy texture and preventing the growth of ice crystals. In drilling muds; in coatings; in the flocculation of solids in water treatment; as sizing agent; thickener; emulsion stabilizer; suspending agent in soft drinks; in dental impression preparations. Pharmaceutic aid (suspending agent).Общее описание
Alginic acid sodium is a gelling and nontoxic anionic polysaccharide. The carboxylic acid groups on the alginic acid chain, renders it insoluble in water.However, converting alginic acid to its sodium form, enables it to solubilize in water easily.Профиль безопасности
Poison by intravenous and intraperitoneal routes. When heated to decomposition it emits toxic fumes of Na2OБезопасность
Sodium alginate is widely used in cosmetics, food products, and pharmaceutical formulations, such as tablets and topical products, including wound dressings. It is generally regarded as a nontoxic and nonirritant material, although excessive oral consumption may be harmful. A study in five healthy male volunteers fed a daily intake of 175 mg/kg body-weight of sodium alginate for 7 days, followed by a daily intake of 200 mg/kg body-weight of sodium alginate for a further 16 days, showed no significant adverse effects.TheWHOhas not specified an acceptable daily intake for alginic acid and alginate salts as the levels used in food do not represent a hazard to health.
Inhalation of alginate dust may be irritant and has been associated with industrial-related asthma in workers involved in alginate production. However, it appears that the cases of asthma were linked to exposure to seaweed dust rather than pure alginate dust.
LD50 (cat, IP): 0.25 g/kg
LD50 (mouse, IV): 0.2 g/kg
LD50 (rabbit, IV): 0.1 g/kg
LD50 (rat, IV): 1 g/kg
LD50 (rat, oral): >5 g/kg
хранилище
Sodium alginate is a hygroscopic material, although it is stable if stored at low relative humidities and a cool temperature.Aqueous solutions of sodium alginate are most stable at pH 4–10. Below pH 3, alginic acid is precipitated. A 1% w/v aqueous solution of sodium alginate exposed to differing temperatures had a viscosity 60–80% of its original value after storage for 2 years.) Solutions should not be stored in metal containers.
Sodium alginate solutions are susceptible on storage to microbial spoilage, which may affect solution viscosity. Solutions are ideally sterilized using ethylene oxide, although filtration using a 0.45 mm filter also has only a slight adverse effect on solution viscosity.
Heating sodium alginate solutions to temperatures above 70°C causes depolymerization with a subsequent loss of viscosity. Autoclaving of solutions can cause a decrease in viscosity, which may vary depending upon the nature of any other substances present. Gamma irradiation should not be used to sterilize sodium alginate solutions since this process severely reduces solution viscosity.
Preparations for external use may be preserved by the addition of 0.1% chlorocresol, 0.1% chloroxylenol, or parabens. If the medium is acidic, benzoic acid may also be used.
The bulk material should be stored in an airtight container in a cool, dry place.
Методы очистки
Free it from heavy metal impurities by treatment with ion-exchange resins (Na+-form), or with a dilute solution of the sodium salt of EDTA. Alternatively dissolve it in 0.1M NaCl, centrifuge and fractionally precipitate it by gradual addition of EtOH or 4M NaCl. The resulting gels are centrifuged off, washed with aqueous EtOH or acetone, and dried under vacuum. [Büchner et al. J Chem Soc 3974 1961.] Sodium n-alkylsulfates. Recrystallise these salts from EtOH/Me2CO [Hashimoto & Thomas J Am Chem Soc 107 4655 1985].Несовместимости
Sodium alginate is incompatible with acridine derivatives, crystal violet, phenylmercuric acetate and nitrate, calcium salts, heavy metals, and ethanol in concentrations greater than 5%. Low concentrations of electrolytes cause an increase in viscosity but high electrolyte concentrations cause salting-out of sodium alginate; salting-out occurs if more than 4% of sodium chloride is present.Регуляторный статус
GRAS listed. Accepted in Europe for use as a food additive. Included in the FDA Inactive Ingredients Database (oral suspensions and tablets). Included as an excipient in nonparenteral medicines (oral capsules, modified release tablets, enteric-coated tablets and lozenges) licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.использованная литература
Pawar, Siddhesh N., and Kevin J. Edgar. Biomaterials 33.11 (2012): 3279-3305. Yang, Ji-Sheng, Ying-Jian Xie, and Wen He. Carbohydrate polymers 84.1 (2011): 33-39.Альгиновая кислота натриевая сол запасные части и сырье
Альгиновая кислота натриевая сол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618007136271 | China | 5999 | 58 | ||
| +86-0086-025-69916489 +86-18852044786 |
China | 323 | 58 | ||
| +8613031735486 | China | 105 | 58 | ||
| +86-15531157085 +86-15531157085 |
China | 8808 | 58 | ||
| +8617531153977 | China | 5855 | 58 | ||
| +86-13131129325 | China | 5872 | 58 | ||
| +86-0311-87836622 +86-18712993135 |
China | 8051 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +86-15532196582 +86-15373005021 |
China | 3007 | 58 | ||
| +8617756083858 | China | 973 | 58 |