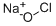
Натрия гипохлорит
- английское имяSodium hypochlorite
- CAS №7681-52-9
- CBNumberCB1705333
- ФормулаClNaO
- мольный вес74.44
- EINECS231-668-3
- номер MDLMFCD00011120
- файл Mol7681-52-9.mol
| Температура плавления | -16 °C |
| Температура кипения | 111 °C |
| плотность | 1.25 g/mL at 20 °C |
| давление пара | 17.5 mmHg ( 20 °C) |
| показатель преломления | 1.3870 |
| температура хранения | 2-8°C |
| растворимость | Methanol (Slightly), Water (Slightly) |
| форма | Solution |
| цвет | Light yellow |
| Удельный вес | 1.209 |
| Запах | pale greenish to yel. liq., chlorine bleach odor |
| РН | 12-13 (20°C, 68 °F) |
| Растворимость в воде | decomposes. |
| Мерк | 14,8628 |
| Диэлектрическая постоянная | 6.7(Ambient) |
| Пределы воздействия | ACGIH: Ceiling 2 mg/m3 OSHA: TWA 2 mg/m3 NIOSH: IDLH 10 mg/m3; Ceiling 2 mg/m3 |
| BCS Class | 1 |
| Стабильность | Stable. Contact with acids releases poisonous gas ( chlorine ). Light sensitive. Incompatible with strong acids, amines, ammonia, ammonium salts, reducing agents, metals, aziridine, methanol, formic acid, phenylacetonitrile. |
| LogP | -3.42 at 20℃ |
| Справочник по базе данных CAS | 7681-52-9(CAS DataBase Reference) |
| Вещества, добавляемые в пищу (ранее EAFUS) | SODIUM HYPOCHLORITE |
| FDA 21 CFR | 173.315; 175.105; 176.170; 177.2800 |
| Рейтинг продуктов питания EWG | 3 |
| FDA UNII | DY38VHM5OD |
| Словарь наркотиков NCI | Dakin's solution |
| Код УВД | D08AX07 |
| Пестициды Закон о свободе информации (FOIA) | Sodium hypochlorite |
| Система регистрации веществ EPA | Sodium hypochlorite (7681-52-9) |
| UNSPSC Code | 51473704 |
| NACRES | NA.21 |
| Коды опасности | C,Xi,N | |||||||||
| Заявления о рисках | 31-34-36/38-36/37/38-50 | |||||||||
| Заявления о безопасности | 26-36/37/39-45-50A-28A-36-61-50-28 | |||||||||
| РИДАДР | UN 1791 8/PG 3 | |||||||||
| WGK Германия | 2 | |||||||||
| RTECS | NH3486300 | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 28289000 | |||||||||
| Банк данных об опасных веществах | 7681-52-9(Hazardous Substances Data) | |||||||||
| Токсичность | Skin contact with the solid hypochlorite pentahydrate or its concentrated solution can cause irritation. Ingestion may cause corrosion of mucous membranes and gastric perforation. | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P363:Перед повторным использованием выстирать загрязненную одежду.
Натрия гипохлорит химические свойства, назначение, производство
Химические свойства
Sodium hypochlorite, NaOCl, is an air-unstable,pale green crystalline solid that is soluble in cold water, decomposes in hot water, and has a sweet aroma. It generally is available in one of two strengths. The household liquid bleach contains about 5.25 wt% NaCIO. The commercial product(sometimes called 15% bleach) contains 150g/L available chlorine. This is equivalent to about 13 wt% sodium hypochlorite. Sodium hypochlorite is used as a bleaching agent for paper pulp and textiles, as an oxidizing reagent, as a disinfectant, as a chemical intermediate,and in medicines.The hypochlorite ion (OCI-) is similar to wet chlorine gas in its effects on materials. Not many metals exhibit good resistance even at low temperatures and concentrations. Because hypochlorite solutions are unstable at neutral and lower pHs,they normally contain excess alkali,which modifies the aggressiveness somewhat.
Физические свойства
Anhydrous sodium hypochlorite explodes; the pentahydrate is a pale-green crystalline solid; orthorhombic structure; density 1.6 g/cm3; melts at 18°C; decomposed by CO2 in the air; soluble in water, 29.3 g/100 mL at 0°C; the aqueous solution is highly stable.История
Sodium hypochlorite exists as an aqueous solution from 5 15% NaOCl and is commonly called bleach. Household bleach is typically a 5.25% solution, and industrial bleach is sold as a 12% solution. When sodium hypochlorite is used in this entry, it is assumed to be the aqueous solution, which is clear, slightly yellow, corrosive, and has a distinctive chlorine smell. Chorine gas was discovered by Carl Wilhelm Scheele (1742 1786) in 1774 and known initially as depholgisticated salt spirit. In 1787, the French chemist Claude Louis Berthollet (1749 1822) experimented with aqueous solution of chlorine gas as bleaching agents. Based on Berthollet's work, the Javel Company located on the outskirts of Paris began to produce bleaches in 1788. Chlorine gas was dissolved in a solution of soda potash (potassium carbonate) to obtain a product called liqueur de Javel, which was potassium hypochlorite. Potash treated with chlorine gas was also used to produce bleaching powders. In 1820, Antoine Germaine Labarraque (1777 1850), an apothecary, substituted cheaper soda ash (sodium carbonate) for potash to produce Eau de Labarraque or Labarraque solution, which was sodium hypochlorite. Eau de Labarraque was used as a disinfectant and to bleach paper. Bleaching powders, borax, lye, and blueing were used as bleaches throughout the 19th century.Sodium hypochlorite is the primary hypochlorite used as a bleach and disinfectant, accounting for 83% of world hypochlorite use, with calcium hypochlorite accounting for the remaining 17%. Approximately 1 million tons of sodium hypochlorite was used globally in 2005, with about half this amount used in households for laundry bleaching and disinfection. The other half was used primarily for wastewater and drinking water treatment; other uses include pool sanitation, bleaching of pulp, paper, and textiles, and as an industrial chemical.
Использование
Sodium hypochlorite is marketed only as an aqueous solution because the anhydrous solid is highly unstable and can explode. The solid pentahydrate also is unstable in air, decomposed by reaction with carbon dioxide from air. Aqueous solutions are very stable. They are used for bleaching textiles and paper pulp; in cleaning solutions; in water purification; as a disinfectant for swimming pools; and as a germicide and topical antiinfective. The hypochlorite also is used as an oxidizing agent in many preparative reactions. It is an ingredient of commercial bleaching products such as Clorox and Dazzle.Подготовка
Sodium hypochlorite solution is obtained by passing chlorine into sodium hydroxide solution. The pentahydrate is obtained by crystallization.Определение
ChEBI: An inorganic sodium salt in which hypochlorite is the counterion.Общее описание
Green to yellow watery liquid with an odor of bleaching liquid odor. Sinks and mixes with water.Реакции воздуха и воды
Water soluble. Decomposes into chlorine and oxygen gases in hot water.Профиль реактивности
Salts of hypochlorous acid, HClO. Generally toxic, irritants and powerful oxidizers, particularly in the presence of water at higher temperature as they decompose to release oxygen and chlorine gases. On contact with urea they form the highly explosive NCl3 . When heated or on contact with acids, they produce highly toxic fumes of chlorine gas [Sax, 9th ed., 1996, p. 1905]. Can react with sulfuric acid to produce heat and chlorine gas.Опасность
Fire risk in contact with organic materials. Toxic by ingestion, strong irritant to tissue.Угроза здоровью
Liquid can be irritating to skin and eyes if contact is maintained.Пожароопасность
Behavior in Fire: May decompose, generating irritating chlorine gas.Побочные эффекты
Sodium hypochlorite, commonly known as bleach, may be used as a disinfectant solution. It is a strong irritant; however, isolated reports of CoU to sodium hypochlorite exist. The mechanism for the Cou is uncertain.Hostynek et al. describe a 36-year-old woman who developed an intensely pruritic maculopapular rash to a hypochlorite-containing cleaning product that she spilled on her leg. The rash progressed to involve her trunk and extremities and was associated with teary eyes, dyspnea, and facial edema. There was a history of a previous sensitizing event, and open testing to 1% sodium hypochlorite produced an immediate urticarial reaction. The authors suggest that this could be due to an immunological mechanism given the generalized symptoms; however, no confirmatory testing was performed and the potential of sodium hypochlorite to cause nonimmunologic Cou was evident with four of 10 controls experiencing a wheal-and-flare reaction to open application of 6% sodium hypochlorite.
Caliskan et al. described a 32-year-old female who developed severe lip edema and breathing difficulty after using a sodium hypochlorite irrigation during endodontic treatment. A scratch test to sodium hypochlorite resulted in immediate erythema and edema that began to extend up the patient’s arm. She also had a history of breathing difficulties and had developed dermatitis from her hands to elbows with the use of household cleaning agents.
Neering reported on a patient who had experienced intermittent Cou to chlorinated pools and contact with a cleansing agent containing sodium hypochlorite. A scratch test to chlorinated water was strongly positive in this patient, but negative in five controls, and closed patch testing to sodium hypochlorite was strongly positive at three hours.
Профиль безопасности
Mddly toxic by ingestion. Human systemic effects by ingestion: somnolence, blood pressure lowering, corrosive to skin, nausea or vomiting. Human mutation data reported. An eye irritant. Corrosive and irritating by ingestion and inhalation. The anhydrous salt is highly explosive and sensitive to heat or friction. Explosive reaction with formic acid (at So), phenylacetonitrile. Reacts to form explosive products with amines, ammonium salts (e.g., ammonium acetate, (NH4)2CO3, ammonium nitrate, ammonium oxalate, (NH4)3P04), aziridme, methanol. Violent reaction with phenyl acetonitrile, cellulose, ethyleneimine. Solutions in water are storage hazards due to oxygen evolution. When heated to decomposition it emits toxic fumes of NaaO and Cl-. Used as a bleach.Натрия гипохлорит запасные части и сырье
сырьё
запасной предмет
- 3,3-Dimethyl-2-oxobutyric acid
- 4-CHLORO-1H-PYRAZOLE-3-CARBOXYLIC ACID
- 3-ХЛОР-6-НИТРО (1H)ИНДАЗОЛ
- chlorinated sodium phosphate
- Этиловый антранила
- 6-АМИНО-3-ХЛОРО (1H) ИНДАЗОЛ
- IMMEDIAL BRILLIANT GREEN GB
- 4,6-диметил-пиримидин-2-сульфонил фторид
- 1-Амино-2 ,4-dibromoanthraquinone
- 4-хлоримидазол
- 4-Morpholineethanesulfonic acid
- 1,3-дихлор-5 ,5-диметилгидантоин
- Magentagreencrystals
- Direct Fast Yellow RR
- Бенз [cd] индол-2 (1H) -он
- 2,5-Dichloronicotinic acid
- DIRECT YELLOW 29
- 5-CHLOROPYRIDIN-2(1H)-ONE
- Рутений(III) хлорид
- 1-хлорбензотризол
- 4-МЕТИЛ-ПИРИМИДИН-2-СУЛЬФОНИЛФТОРИД
- 2- (морфолинотио) бензотиазол
- АТРАЗИН
- MEQUINDOX
- 3,3-Dimethylglutaric кислота
- 3-метилтиофен-2-карбонилхлорид
- ЙОДОФОРМ
- 4-ХЛОР-3-МЕТИЛ-1H-ПИРАЗОЛ
- Chiniofon
- 2-CHLOROSULFONYL-PYRIDINIUM, CHLORIDE
1of8
Натрия гипохлорит поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +86-15531157085 +86-15531157085 |
China | 8808 | 58 | |
| +8617736087130 | China | 994 | 58 | |
| +86-86-02137122233 +8613795318958 |
China | 299 | 55 | |
| +86-0371-55170693 +86-19937530512 |
China | 21630 | 55 | |
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | |
| +86-13734021967 +8613734021967 |
China | 1001 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29862 | 58 | |
| +86 18953170293 | China | 2930 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| +86-86-5926051114 +8615060885618 |
China | 6383 | 58 |