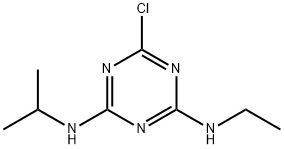
АТРАЗИН
- английское имяAtrazine
- CAS №1912-24-9
- CBNumberCB6349448
- ФормулаC8H14ClN5
- мольный вес215.68
- EINECS217-617-8
- номер MDLMFCD00041810
- файл Mol1912-24-9.mol
| Температура плавления | 175°C |
| Температура кипения | 200°C |
| плотность | 1.187 |
| давление пара | 0Pa at 25℃ |
| показатель преломления | 1.6110 (estimate) |
| Fp | 11 °C |
| температура хранения | Keep in dark place,Inert atmosphere,Room temperature |
| растворимость | DMSO: 83.33 mg/mL (386.36 mM) |
| пка | pKa 1.64 (Uncertain) |
| форма | Crystalline |
| цвет | Crystals |
| Растворимость в воде | Slightly soluble. 0.007 g/100 mL |
| Мерк | 14,871 |
| БРН | 612020 |
| Пределы воздействия | OSHA PEL: TWA 5 mg/m3; ACGIH TLV: TWA 5 mg/m3. |
| Стабильность | Stable. Incompatible with strong oxidizing agents. |
| Стандарт первичной питьевой воды EPA | MCL:0.003,MCLG:0.003 |
| LogP | 2.59 at 20℃ and pH7.31-7.51 |
| Surface tension | 57.6mN/m at 30mg/L and 21℃ |
| Dissociation constant | 1.56 at 20℃ |
| FDA 21 CFR | 165.113 |
| Справочник по базе данных CAS | 1912-24-9(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 7 |
| FDA UNII | QJA9M5H4IM |
| Предложение 65 Список | Atrazine |
| МАИР | 3 (Vol. 53, 73) 1999 |
| Справочник по химии NIST | Atrazine(1912-24-9) |
| Система регистрации веществ EPA | Atrazine (1912-24-9) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | Xn;N,N,Xn,T,F,Xi | |||||||||
| Заявления о рисках | 43-48/22-50/53-39/23/24/25-23/24/25-11-38-36/37/38-20/21/22-52/53 | |||||||||
| Заявления о безопасности | 2-36/37-60-61-45-16-7-36-26 | |||||||||
| РИДАДР | 3077 | |||||||||
| OEB | B | |||||||||
| OEL | TWA: 5 mg/m3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | XY5600000 | |||||||||
| Класс опасности | 9 | |||||||||
| Группа упаковки | III | |||||||||
| кода HS | 29336990 | |||||||||
| Банк данных об опасных веществах | 1912-24-9(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in mice: 1750 mg/kg (Dalgaard-Mikkelsen, Poulsen) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
предупреждение
-
вредная бумага
H317:При контакте с кожей может вызывать аллергическую реакцию.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P272:Не уносить загрязненную спецодежду с места работы.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P302+P352:ПРИ ПОПАДАНИИ НА КОЖУ: Промыть большим количеством воды.
P314:В случае плохого самочувствия обратиться к врачу.
АТРАЗИН химические свойства, назначение, производство
Химические свойства
Atrazine is a colorless, crystalline solid. Although atrazine is very stable, it is only slightly soluble in water, but soluble in N-pentane, chloroform, dimethyl sulfoxide, ethyl acetate, diethyl ether, and methanol. Atrazine is a broad-spectrum triazine herbicide and is used as a selective herbicide for weed control in corn and asparagus, in the culture of sugarcane and pineapple. Additionally, it is used as a total herbicide on roads and public places as well as on uncultivated ground in combination with amitrol, bromacil, dalapon, and growth promoters. Atrazine inhibits photosynthesis and other metabolic processes in plants. There are no natural sources of atrazine. It is produced from cyanuric acid chloride with ethylamine and isopropylamine. The reaction takes place successively in tetrachloromethane. All atrazine produced is released into the environment. The formulations include granules, water dispersible granules, liquid, suspension concentrate, wettable powder, and a combination with many other herbicides. Atrazine is compatible with various insecticides and fungicides.Atrazine was banned in the European Union (EU) in 2004 because of its persistent groundwater contamination. In the United States, however, atrazine is one of the most widely used herbicides, with 76 million pounds of it applied each year. It is probably the most commonly used herbicide in the world.
Использование
Atrazine is widely used as a selective herbicide to control broadleaf and grassy weeds in corn, sorghum, rangeland, sugarcane, orchards, pineapple, and turf grass sod. It is also used for selective weed control in conifer restoration and Christmas tree plantations. It is also used as a nonselective herbicide for vegetation control in noncrop land.Методы производства
Atrazine is prepared by reacting cyanuric chloride with one equivalent of ethylamine, followed by one equivalent of isopropylamine in the presence of an acid-binding agent.Определение
ChEBI: A diamino-1,3,5-triazine that is 1,3,5-triazine-2,4-diamine substituted by a chloro group at position 6 while one of hydrogens of each amino group is replaced respectively by an ethyl and a propan-2-yl group.Общее описание
White crystalline solid. Melting point 173-175°C. Sinks in water. A selective herbicide used for season-long weed control in a variety of crops.Реакции воздуха и воды
Insoluble in water.Профиль реактивности
Atrazine undergoes slow hydrolysis at 158° F under neutral conditions. Hydrolysis is more rapid in acidic or alkaline conditions. Forms salts with acids .Опасность
Hematologic, preproductive and develop- mental effects. Questionable carcinogen.Пожароопасность
Special Hazards of Combustion Products: Irritating hydrogen chloride and toxic oxides of nitrogen may be formed.Сельскохозяйственное использование
Atrazine is the generic name for 2-chloro-4-ethylamino- 6-isopropylamino-s-triazine.A trazine is an example of photosynthesis inhibitors and herbicides.Atrazine was the first s-triazine used in maize. The use of this herbicide and others in the same group has expanded to selective application in perennial crops and orchids as well as for non-crop and industrial sites.
Фармаколо?гия
In plants, themajor pathways of atrazine transformation include hydroxylation, N-dealkylation, and glutathione conjugation. Hydroxylation of atrazine and other s-triazines occurs in a wide range of plants and is considered a detoxification mechanism because hydroxyatrazine is not phytotoxic. Hydroxylation is catalyzed via reaction with the naturally occurring compound, benzoxazinone (127). A hypothetical ether-linked intermediate has been proposed to undergoes hydrolysis to produce hydroxylated triazine and regenerated benzoxazinone. This reaction occurs in many susceptible and resistant plant species, and the rate of this reaction is governed by the amount of benzoxazinone present in the tissue. The hydroxylation reaction predominates in root tissue, whereas GSH conjugation is more prominent in leaf tissue of plants containing a GST that has substrate specificity for atrazine. Because atrazine is a photosystem II (PS II) inhibitor, possession of a rapid detoxification system, such as a specific GST, is paramount to provide tolerance to this compound.Профиль безопасности
Poison by intraperitoneal route. Moderately toxic by ingestion. Mildly toxic by inhalation and skin contact. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A skin and severe eye irritant. Questionable carcinogen with experimental tumorigenic data. When heated to decomposition it emits toxic fumes of ClandNOx.Возможный контакт
Atrazine is an herbicide and plant growth regulator used for season-long weed control in corn, sorghum, and certain other crops. Banned for use as a pesticide in the EU. US annual use . 75 millon pounds.Канцерогенность
An increase in mammary adenomas and fibroadenomas was observed in female rats fed 1000 ppm, but not 500 ppm atrazine or less for their lifetime. An increase in the incidence of mammary carcinomas was seen at 70, 500, and 1000 ppm, but not at 10 ppm. The biological significance of these findings is not known but may be related to a hormonally mediated mechanism. Rats fed 375 or 750 ppmatrazine for 2 years showed an increase in mammary tumors in males at 750 ppm. Uterine carcinomas increased in both groups and the incidence of malignant tumors also increased in both sexes.Экологическая судьба
Atrazine is highly persistent in soil. In soil and water, atrazine degrades by hydrolysis, followed by biodegradation by soil microorganisms. Hydrolysis is rapid in acidic or basic environments, but is slower around neutral pH. Sunlight and evaporation do not affect its removal rate. Atrazine can persist for longer than 1 year under dry or cold conditions. It is moderately to highly mobile in soils with low clay or low organic matter content. Because it does not adsorb strongly to soil particles and has a lengthy half-life (60 to >100 days), it has a high potential for groundwater contamination despite being only moderately soluble in water. It is frequently detected in drinking water wells.Перевозки
UN2763 Triazine pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.Несовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.Утилизация отходов
Atrazine is hydrolyzed by either acid or base. The hydroxy compounds are generally herbicidally inactive, but their complete environmental effects are uncertain. However, the method appears suitable for limited use and quantities of triazine. Atrazine underwent .99% decomposition when burned in a polyethylene bag, and combustion with a hydrocarbon fuel would appear to be a generally suitable method for small quantities. Combustion of larger quantities would probably require the use of a caustic wet scrubber to remove nitrogen oxides and HCl from the product gases.АТРАЗИН запасные части и сырье
АТРАЗИН поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| +8613573296305 | China | 301 | 58 | |
| +8613288715578 | China | 1174 | 58 | |
| +8615350851019 | China | 1001 | 58 | |
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | |
| 008657128800458; +8615858145714 |
China | 7738 | 55 | |
| +undefined-21-51877795 | China | 32965 | 60 | |
| 18017610038 | CHINA | 3619 | 58 | |
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | |
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 |