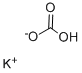
Калия гидрокарбонат
- английское имяPotassium bicarbonate
- CAS №298-14-6
- CBNumberCB3260704
- ФормулаCHKO3
- мольный вес100.12
- EINECS206-059-0
- номер MDLMFCD00011402
- файл Mol298-14-6.mol
| Температура плавления | 292 °C |
| плотность | 2,17 g/cm3 |
| температура хранения | Store at +15°C to +25°C. |
| растворимость | H2O: 1 M at 20 °C, clear, colorless |
| форма | fine crystals |
| пка | 10.33(at 25℃) |
| цвет | White |
| Удельный вес | 2.17 |
| Запах | Odorless |
| Водородный показатель | 8 - 9 |
| РН | 8.27(1 mM solution);8.25(10 mM solution);8.13(100 mM solution); |
| Растворимость в воде | Soluble in water. Insoluble in alcohol. |
| λмакс | λ: 260 nm Amax: 0.010 λ: 280 nm Amax: 0.01 |
| Мерк | 14,7609 |
| БРН | 4535309 |
| Стабильность | Stable. |
| ИнЧИКей | TYJJADVDDVDEDZ-UHFFFAOYSA-M |
| Справочник по базе данных CAS | 298-14-6(CAS DataBase Reference) |
| FDA 21 CFR | 184.1613; 582.1613; 310.545 |
| Вещества, добавляемые в пищу (ранее EAFUS) | POTASSIUM BICARBONATE |
| Специальный комитет по веществам GRAS | Potassium bicarbonate |
| Рейтинг продуктов питания EWG | 1 |
| FDA UNII | HM5Z15LEBN |
| Код УВД | A12BA04 |
| Система регистрации веществ EPA | Potassium bicarbonate (298-14-6) |
| UNSPSC Code | 41116107 |
| NACRES | NB.24 |
| Заявления о рисках | 22-35 | |||||||||
| Заявления о безопасности | 24/25-45-36/37/39-26 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | FG1840000 | |||||||||
| F | 3 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 28364000 | |||||||||
| Токсичность | LD50 orally in Rabbit: > 2000 mg/kg | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H335:Может вызывать раздражение верхних дыхательных путей.
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P261:Избегать вдыхания пыли/ дыма/ газа/ тумана/ паров/ аэрозолей.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Калия гидрокарбонат химические свойства, назначение, производство
Химические свойства
Potassium bicarbonate occurs as colorless, transparent crystals or as a white granular or crystalline powder. It is odorless, with a saline or weakly alkaline taste.Использование
Potassium Bicarbonate is an alkali and leavening agent obtained as colorless prisms or white powder. it is very soluble, with 1 g dis- solving in 2.8 ml of water. upon heating, it liberates carbon dioxide which provides leavening in baked goods. it is also used in confec- tionary products.Методы производства
Potassium bicarbonate can be made by passing carbon dioxide into a concentrated solution of potassium carbonate, or by exposing moist potassium carbonate to carbon dioxide, preferably under moderate pressure.Potassium bicarbonate also occurs naturally in the mineral calcinite.
Определение
ChEBI: A potassium salt that is the monopotassium salt of carbonic acid. It has fungicidal properties and is used in organic farming for the control of powdery mildew and apple scab.Общее описание
Potassium bicarbonate is water soluble alkaline potassium salt with monoclinic crystalline structure. It is a raw material for the synthesis of many potassium compounds. It is a better coolant than sodium bicarbonate in the aerosol fire extinguishing apparatus. It shows potential as an antifungal agent.Сельскохозяйственное использование
Potassium bicarbonate (KHCO3), also called potassium hydrogen carbonate, is a white crystalline solid, soluble in water (insoluble in ethanol). It decomposes at about 120°C. Potassium bicarbonate contains about 28% potassium (K2O) and used as a potassium supplying fertilizer.Potassium bicarbonate, which occurs naturally as calcinite, is made by passing carbon dioxide into saturated potassium carbonate solution. It is used as baking powder and as a fire extinguisher.
Фармацевтические приложения
As an excipient, potassium bicarbonate is generally used in formulations as a source of carbon dioxide in effervescent preparations, at concentrations of 25–50% w/w. It is of particular use in formulations where sodium bicarbonate is unsuitable, for example, when the presence of sodium ions in a formulation needs to be limited or is undesirable. Potassium bicarbonate is often formulated with citric acid or tartaric acid in effervescent tablets or granules; on contact with water, carbon dioxide is released through chemical reaction, and the product disintegrates. On occasion, the presence of potassium bicarbonate alone may be sufficient in tablet formulations, as reaction with gastric acid can be sufficient to cause effervescence and product disintegration.Potassium bicarbonate has also been investigated as a gasforming agent in alginate raft systems.The effects of potassium bicarbonate on the stability and dissolution of paracetamol and ibuprofen have been described.
Potassium bicarbonate is also used in food applications as an alkali and a leavening agent, and is a component of baking powder. Therapeutically, potassium bicarbonate is used as an alternative to sodium bicarbonate in the treatment of certain types of metabolic acidosis. It is also used as an antacid to neutralize acid secretions in the gastrointestinal tract and as a potassium supplement.
Безопасность
Potassium bicarbonate is used in cosmetics, foods, and oral pharmaceutical formulations, where it is generally regarded as a relatively nontoxic and nonirritant material when used as an excipient. However, excessive consumption of potassium bicarbonate or other potassium salts may produce toxic manifestations of hyperkalemia.хранилище
Potassium bicarbonate should be stored in a well-closed container in a cool, dry location. Potassium bicarbonate is stable in air at normal temperatures, but when heated to 100–200°C in the dry state, or in solution, it is gradually converted to potassium carbonate.Методы очистки
It is crystallised from water at 65-70o (1.25mL/g) by filtering and then cooling to 15o (~0.4ml/g). During all operations, CO2 is passed through the stirred mixture. The crystals are sucked dry at the pump, washed with distilled water, dried in air and then over H2SO4 in an atmosphere of CO2. It is much less soluble than the carbonate in H2O (see below).Несовместимости
Potassium bicarbonate reacts with acids and acidic salts with the evolution of carbon dioxide.Регуляторный статус
E501 refers to potassium carbonates). Included in nonparenteral medicines licensed in the UK and USA (chewable tablets; effervescent granules; effervescent tablets; lozenges; oral granules; oral suspensions; powder for oral solutions). Included in the Canadian List of Acceptable Non-medicinal Ingredients.Калия гидрокарбонат запасные части и сырье
сырьё
1of2
запасной предмет
- 2-БЕНЗАМИДО-3- (4- (N, N-БИС- (2-ХЛОРОЭТИЛ) АМИНО) ФЕНИЛ) ПРОПИОНОВАЯ КИСЛОТА
- Азтреонам
- Methyl 7-amino-3-methyl-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate
- Ленампициллин
- N-альфа-BOC-N-эпсилон-(2-хлор-Z)-L-лизин
- Ethyl 3-bromo-2-(bromomethyl)propionate
- 4-(4-(BIS-(2-CHLOROETHYL)AMINO)BENZYLIDENE-2-PHENYL-OXAZOLINE-5-ONE
- Калий тетрафтороборат
- H-LYS(2-CL-Z)-OH
- CILAZAPRIL
- арбонат калия
- Калий метабисульфит
- Cefpiramide acid
- Potassium sodium tartrate
- 1-Вос-азетидин-3-карбоксальдегида
1of5
Калия гидрокарбонат поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +8618805133257 | China | 132 | 60 | ||
| +86-86-17798073498 +8617798073498 |
China | 299 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +8617732866630 | China | 18147 | 58 | ||
| +86-17331933971 +86-17331933971 |
China | 2472 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 |