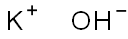
Гидроксид калия
- английское имяPotassium hydroxide
- CAS №1310-58-3
- CBNumberCB3107908
- ФормулаKOH
- мольный вес56.11
- EINECS215-181-3
- номер MDLMFCD00003553
- файл Mol1310-58-3.mol
| Температура плавления | 361 °C (lit.) |
| Температура кипения | 1320°C |
| плотность | 1.450 g/mL at 20 °C |
| давление пара | 1 mm Hg ( 719 °C) |
| показатель преломления | n |
| Fp | 52 °F |
| температура хранения | Store at +5°C to +30°C. |
| растворимость | H2O: 1 M at 20 °C, clear, colorless |
| форма | powder |
| Удельный вес | 1.09 |
| цвет | white |
| Запах | Odorless |
| РН | 10.98(1 mM solution);11.95(10 mM solution);12.88(100 mM solution); |
| Пределы взрываемости | 3.5-15.0%(V) (ethanol) |
| Растворимость в воде | soluble |
| Чувствительный | Air Sensitive & Hygroscopic |
| Мерк | 14,7640 |
| Пределы воздействия | Ceiling in air 2 mg/m3 (ACGIH). |
| Диэлектрическая постоянная | 3.3(Ambient) |
| Стабильность | Stable, but very hygroscopic. Dissolves exothermically in water. Incompatible with most metals, strong acids, acid chlorides, organic materials, zinc, aluminium, nitroalkanes, nitrobenzene, chlorine dioxide. Reacts vigorously with a wide variety of other materials. Readily absorbs water and carbon dioxide from the air. |
| ИнЧИКей | KWYUFKZDYYNOTN-UHFFFAOYSA-M |
| LogP | -1.380 (est) |
| FDA 21 CFR | 184.1631; 582.1631; 175.210; 177.2800 |
| Вещества, добавляемые в пищу (ранее EAFUS) | POTASSIUM HYDROXIDE |
| Специальный комитет по веществам GRAS | Potassium hydroxide |
| Справочник по базе данных CAS | 1310-58-3(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2-5 |
| FDA UNII | WZH3C48M4T |
| Словарь онкологических терминов NCI | potassium hydroxide |
| Справочник по химии NIST | Potassium hydroxide(1310-58-3) |
| Система регистрации веществ EPA | Potassium hydroxide (1310-58-3) |
| Информация о косметике | Potassium Hydroxide |
| UNSPSC Code | 12352320 |
| NACRES | NB.21 |
| Коды опасности | C,F,T,Xi | |||||||||
| Заявления о рисках | 34-35-22-11-39/23/24/25-23/24/25-36/38-36/37-67-52/53 | |||||||||
| Заявления о безопасности | 7-16-36/37-45-36/37/39-26-61 | |||||||||
| РИДАДР | UN 2924 3/PG 2 | |||||||||
| OEL | Ceiling: 2 mg/m3 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | TT2100000 | |||||||||
| F | 3 | |||||||||
| Температура самовоспламенения | 425 °C (ethanol) | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 28259090 | |||||||||
| Банк данных об опасных веществах | 1310-58-3(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in rats: 1.23 g/kg (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
опасность
-
вредная бумага
H302:Вредно при проглатывании.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H290:Может вызывать коррозию металлов.
-
оператор предупредительных мер
P234:Хранить только в оригинальной упаковке.
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
Гидроксид калия химические свойства, назначение, производство
Химические свойства
Potassium hydroxide occurs as a white or nearly white fused mass. It is available in small pellets, flakes, sticks and other shapes or forms. It is hard and brittle and shows a crystalline fracture. Potassium hydroxide is hygroscopic and deliquescent; on exposure to air, it rapidly absorbs carbon dioxide and water with the formation of potassium carbonate. Soluble in water, alcohol, glycerol; slightly soluble in ether.Показания
Potassium hydroxide (KOH) is a strong alkali that digests proteins and epidermal debris. In one study, 10% solution was applied b.i.d. to each lesion for 30 days with excellent clearance. The side effects included stinging of the lesion and one case of secondary infection. Also reported were the occurrence of a hypertrophic scar as well as some persistent or transitory hyper- and hypopigmentation. The same authors who used the 5% KOH solution completed further studies and they found it to be as effective-yet with decreased side effects.Методы производства
Potassium hydroxide is made by the electrolysis of potassium chloride. Commercial grades may contain chlorides as well as other impurities.прикладной
Potassium hydroxide is used as an emulsifier in lotions and as an alkali in liquid soaps, protective creams, and shaving preparations. Depending on the concentration used, it can be highly irritating to the skin and/or cause a burning sensation. It is used in making potassium salts, in electroplatingand lithography, in printing inks, as a mordantfor wood, and finds wide applicationsin organic syntheses and chemical analyses.Общее описание
A white solid. Corrosive to metals and tissue. Used in soap manufacture, bleach, as an electrolyte in alkaline batteries, and as a food additive.Реакции воздуха и воды
Hydrolysis generates enough heat to ignite adjacent combustible material [Haz. Chem. Data 1966]. Dissolves in water (with liberation of heat, may steam and spatter. Solution is basic (alkaline). DeliquescentПрофиль реактивности
POTASSIUM HYDROXIDE absorbs moisture readily forming caustic solution that attacks aluminum and zinc. A piece of potassium hydroxide causes liquid chlorine dioxide to explode [Mellor 2:289. 1946-47]. 1,2-dichloroethylene and potassium hydroxide forms chloroacetylene, which is explosive and spontaneously flammable in air. Potassium hydroxide is highly toxic [Rutledge 1968. p. 134]. A reaction between n-nitrosomethylurea and potassium hydroxide in n-butyl ether resulted in an explosion due to the formation of diazomethane [Schwab 1972]. Potassium persulfate and a little potassium hydroxide and water ignited a polythene (polyethylene) liner of a container by release of heat and oxygen [MCA Case History 1155. 1955]. Using potassium hydroxide to dry impure tetrahydrofuran, which contains peroxides, may be hazardous. Explosions have occurred in the past. Sodium hydroxide behaves in a similar way as potassium hydroxide [NSC Newsletter Chem. Soc. 1967]. A strong base. Forms caustic solution in water. [Merck 11th ed. 1989].Угроза здоровью
Toxic by ingestion and inhalation, strong caustic, handle with gloves or tongs, corrosive to tissue. Eye, skin and upper respiratory tract irritant.Potassium hydroxide is a strongly alkaline, hydrophilic substance and therefore solid potassium hydroxide is highly corrosive. It reacts with fat and can cause irreversible damage to any site of contact with the body (for example skin or eyes). Solutions of potassium hydroxide in water at concentrations above 0.5% (w/w) are irritating at points of contact and, at higher concentrations, the solutions can be corrosive. Potassium hydroxide does not cause skin allergies. Because of the corrosive properties of potassium hydroxide, its ingestion can be fatal. Under normal conditions of handling and use, potassium hydroxide in solution will dissociate into its constituent ions and, if ingested, will not be systemically available in the body as such.
Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.Воспламеняемость и взрывоопасность
Sodium hydroxide and potassium hydroxide are not flammable as solids or aqueous solutions.Фармацевтические приложения
Potassium hydroxide is widely used in pharmaceutical formulations to adjust the pH of solutions. It can also be used to react with weak acids to form salts.Therapeutically, potassium hydroxide is used in various dermatological applications.
Профиль безопасности
Poison by ingestion. An eye irritant and severe human skin irritant. Very corrosive to the eyes, skin, and mucous membranes. Mutation data reported. Ingestion may cause violent pain in throat and epigastrium, hematemesis, collapse. Stricture of esophagus may result if substance is not immedately fatal. Above 84' it reacts with reducing sugars to form poisonous carbon monoxide gas. Violent, exothermic reaction with water. Potentially explosive reaction with bromoform + crown ethers, chlorine dioxide, nitrobenzene, nitromethane, nitrogen trichloride, peroxidized tetrahydrofuran, 2,4,6- trinitrotoluene. Reaction with ammonium hexachloroplatinate(2-) + heat forms a heat- sensitive explosive product. Violent reaction or ignition under the appropriate condtions with acids, alcohols, p-bis(l,3- dbromoethyl)benzene, cyclopentadene, germanium, hyponitrous acid, maleic anhydride, nitroalkanes, 2-nitrophenol, potassium peroxodisulfate, sugars, 2,2,3,3- tetrafluoropropanol, thorium dicarbide. When heated to decomposition it emits toxic fumes of K2O. See also SODIUM HYDROXIDE.Безопасность
Potassium hydroxide is widely used in the pharmaceutical and food industries and is generally regarded as a nontoxic material at low concentrations. At high concentrations it is a corrosive irritant to the skin, eyes, and mucous membranes.(rat, oral): 0.273 g/kg
Возможный контакт
KOH is generally used as an alkali and in the manufacture of other potassium compounds.хранилище
splash goggles and impermeable gloves should be worn at all times when handling these substances to prevent eye and skin contact. Operations with metal hydroxide solutions that have the potential to create aerosols should be conducted in a fume hood to prevent exposure by inhalation. NaOH and KOH generate considerable heat when dissolved in water; when mixing with water, always add caustics slowly to the water and stir continuously. Never add water in limited quantities to solid hydroxides. Potassium hydroxide should be stored in an airtight, nonmetallic container in a cool, dry place, separated from acids and incompatible substances.Перевозки
UN1814 (solution) & UN1813 (solid); Potassium hydroxide, solid or solution, Hazard class: 8; Labels: 8-Corrosive material.Методы очистки
Its carbonate content can be reduced by rinsing KOH sticks rapidly with water prior to dissolving them in boiled out distilled water. Alternatively, a slight excess of saturated BaCl2 or Ba(OH)2 can be added to the solution which, after shaking well, is set aside so that the BaCO3 is allowed to separate out. Davies and Nancollas [Nature 165 237 1950] rendered KOH solutions carbonate free by ion exchange using a column of Amberlite IR-100 in the OH-form.Несовместимости
Potassium hydroxide is a strong base and is incompatible with any compound that readily undergoes hydrolysis or oxidation. Violent reaction with acids, alcohols, water, metals (when wet), halogenated hydrocarbons; maleic anhydride. Heat is generated if KOH comes in contact with water and carbon dioxide from the air. It should not be stored in glass or aluminum containers, Corrosive to zinc, aluminum, tin and lead in the presence of moisture releasing combustible/explosive hydrogen gas. Can absorb water from air and give off sufficient heat to ignite surrounding combustible materials.Утилизация отходов
Dilute with large volume of water, neutralize and flush to sewerРегуляторный статус
GRAS listed. Accepted for use in Europe in certain food applications. Included in the FDA Inactive Ingredients Database (injections, infusions, and oral capsules and solutions). Included in nonparenteral and parenteral medicines licensed in the UK. Included in the Canadian List of Acceptable Non-medicinal Ingredients.Гидроксид калия запасные части и сырье
сырьё
1of3
запасной предмет
- 4- (БЕНЗИЛТИО) -3-НИТРОБЕНЗАЛЬДЕГИД
- ПОЛИЭТИЛЕН ГЛИКОЛЬ МОНООЛЕЙЛ ЭФИР
- 4-[(2-FURYLMETHYL)THIO]-3-NITROBENZALDEHYDE
- Potassium stearate
- Тартрат калия
- щавелевая кислота, калиевая соль
- Potassium hydrogen phthalate
- 2-аллилциклогексанон
- 2-Amino-4-trifluoromethylbenzonitrile
- Organosilicon water-proofing agent
- 4-Ethoxyphenylacetic кислота
- 2-бром-6-(1H-пиразол-1-ил)пиридин
- D-глюкозамин гидрохлорид
- antistatic agent F695
- 4-(ЦИКЛОГЕКСИЛТИО)-3-НИТРОБЕНЗАЛЬДЕГИД
- 2-Benzylaminopyridine
- Potassium sorbate
- Potassium isobutylxanthate
- 1,2,3-Propanetricarboxylicacid,2-hydroxy-,potassiumsalt
- 3,5-DIBROMO-2-ETHOXYBENZALDEHYDE
- Potassium metavanadate
- Antistatic agent PK
- Калия лактат
- 2-(2,4-DINITROPHENOXY)ETHANOL
- Potassium fluoroaluminate
- 5-Hydroxymethyluracil
- Калия цитрат
- Пиперониловая кислота
- Геллановая десен
- 1,2,3,4-Тетрагидро-9H-пиридо(3,4-B)-индол
1of8
Гидроксид калия поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0551-65418671 +8618949823763 |
China | 34563 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86-13157026678 +86-13157026678 |
China | 52 | 58 | ||
| +undefined18602966907 | China | 997 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +8615530197691 | China | 105 | 58 | ||
| +8618805133257 | China | 132 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-021-57951555 +8617317452075 |
China | 1803 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 |