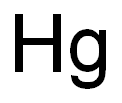
Меркурий
- английское имяMercury
- CAS №7439-97-6
- CBNumberCB7355066
- ФормулаHg
- мольный вес200.59
- EINECS231-106-7
- номер MDLMFCD00011035
- файл Mol7439-97-6.mol
| Температура плавления | -38.9 °C |
| Температура кипения | 356.6 °C(lit.) |
| плотность | 13.54 |
| плотность пара | 7 (vs air) |
| давление пара | <0.01 mm Hg ( 20 °C) |
| температура хранения | Poison room |
| растворимость | H2O: soluble |
| форма | Triple Distilled Liquid |
| Удельный вес | 13.5 (20/4℃) |
| Запах | Odorless |
| Скорость испарения | 4 |
| удельное сопротивление | 95.8 μΩ-cm, 20°C |
| Растворимость в воде | 20–30μg/L in H2O; soluble in boiling H2SO4, HNO3 [KIR81] [HAW93] |
| Мерк | 13,5925 |
| Пределы воздействия | TLV-TWA 0.05 mg/m3 for Hg vapor, and 0.10 mg/m3, as Hg for alkyl mercury and inorganic compounds (ACGIH); ceiling 0.1 mg/m3 (OSHA); IDLH 28 mg/m3 (NIOSH). |
| Диэлектрическая постоянная | 1.0(148℃) |
| Стабильность | Stable. Incompatible with strong acids, sodium thiosulfate, ammonium hydroxide. |
| ИнЧИКей | QSHDDOUJBYECFT-UHFFFAOYSA-N |
| Справочник по базе данных CAS | 7439-97-6(CAS DataBase Reference) |
| FDA 21 CFR | 165.110; 310.545 |
| Словарь онкологических терминов NCI | mercury |
| FDA UNII | FXS1BY2PGL |
| Код УВД | D08AK05 |
| МАИР | 3 (Vol. 58) 1993 |
| Система регистрации веществ EPA | Mercury (7439-97-6) |
| Коды опасности | T,N,Xn,C,T+ | |||||||||
| Заявления о рисках | 25-48/21/22-51/53-50/53-33-23-20/21/22-34-36/37/38-23/24/25-48/23-26-61-52/53-36/38 | |||||||||
| Заявления о безопасности | 7-45-60-61-36-36/37/39-26-36/37-53 | |||||||||
| РИДАДР | UN 3289 6.1/PG 2 | |||||||||
| OEL | STEL: 0.1 mg/m3 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | OV4550000 | |||||||||
| F | 10 | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2805 40 90 | |||||||||
| Класс опасности | 8 | |||||||||
| Группа упаковки | III | |||||||||
| Банк данных об опасных веществах | 7439-97-6(Hazardous Substances Data) | |||||||||
| Токсичность | LCLO inhal (rabbit) 29 mg/m3 (30 h) PEL (OSHA) 0.1 mg/m3 (ceiling) TLV-TWA (ACGIH) 0.025 mg/m3—skin | |||||||||
| ИДЛА | 10 mg Hg/m3 | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H372:Поражает органы в результате многократного или продолжительного воздействия.
H360D:Может отрицательно повлиять на неродившегося ребенка.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
H330:Смертельно при вдыхании.
-
оператор предупредительных мер
P201:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P304+P340+P310:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Немедленно обратиться за медицинской помощью.
Меркурий химические свойства, назначение, производство
Описание
Elemental mercury, a silver-white metal, is also known ‘quicksilver’ or ‘hydrargyrum.’ Mercury has been discovered in Egyptian tombs dating as far back as 1500 BC. The chemical symbol, Hg, is derived from the Greek word hydrargyros, meaning ‘water silver.’ Mercury was known in antiquity and used by alchemists. Its neurological effects were recognized early, and its use in the hat-making trade gave rise to the phrase ‘mad as a hatter.’ Mercury has been used commercially and medically for centuries. In the past it was a common constituent of many medications, for example, it was used in the treatment of syphilis. Use of mercury has been drastically reduced in recent years. Within the twentieth century, mercury used to be in every physician’s or pharmacist’s armamentarium, for example, calomel was commonly used in infant teething powders in the 1930s and 1940s.Химические свойства
Mercury is a silvery, mobile, odorless, extremely heavy liquid , sometimes found native. Insoluble in hydrochloric acid; soluble in sulfuric acid upon boiling; readily soluble in nitric acid; insoluble in water, alcohol, and ether; soluble in lipids; extremely high surface tension.История
The name of Hg derives from the Roman god “Mercury”, the nimble messenger of the gods, since the ancients used that name for the element, which was known from prehistoric times. The name mercury originated in 6th-century alchemy, in which the symbol of the planet was used to represent the metal; the chemical symbol Hg derives from the Latin hydrargyrum, “liquid silver or quick silver.” Although its toxicity was recognized at an early date, its main application was for medical purposes.Использование
Amalgams, catalyst, electrical apparatus, cathodes for production of chlorine and caustic soda, instruments (thermometers, barometers, etc.), mercury vapor lamps, extractive metallurgy, mirror coating, arc lamps, boilers, coolant, and neutron absorber in nuclear power plants.Определение
Metallic element of atomic number 80, group IIB of the periodic table, aw 200.59, valences = 1,2; 4 stable isotopes and 12 artificially radioactive isotopes.Методы производства
Mercury is mined primarily in underground mines as the metal or as the red sulfide cinnabar (HgS). Like HgO, the sulfide decomposes at higher temperatures. Heating of the ore and condensation of the mercury vapor constitute a convenient procedure for reducing, extracting, and purifying mercury from its ore. In the United States, mercury is produced primarily from secondary sources; this involves recycling a variety of industrial waste products. A survey in 1980 conducted by the U.S. National Institute for Occupational Safety and Health suggested that about 70,000 workers were exposed to mercury and its compounds; the majority of these exposures involves mercury vapor. However, this number has probably already decreased considerably, and occupational mercury vapor exposure has now become fairly rare in industrialized countries. On the other hand, numbers of workers exposed to mercury vapor from informal mining in developing countries has increased disproportionally and is causing health risks to workers and their families, including children.Общее описание
An odorless, silvery metallic liquid. Insoluble in water. Toxic by ingestion, absorption and inhalation of the fumes. Corrosive to aluminum. Used as a catalyst in instruments, boilers, mirror coatings.Профиль реактивности
MERCURY forms an explosive acelylide when mixed with acetylene. Can form explosive compounds with ammonia (a residue resulting from such a reaction exploded when an attempt was made to clean MERCURY off a steel rod [Chem. Eng. News 25:2138. 1947]. Chlorine dioxide (also other oxidants, such as: chlorine, bromine, nitric acid, performic acid), and MERCURY explode when mixed [Mellor 2, Supp. 1:381. 1956]. Methyl azide in the presence of MERCURY is potentially explosive [Can. J. Chem. 41:1048. 1963]. Ground mixtures of sodium carbide and MERCURY can react vigorously [Mellor 5:848. 1946-47]. Ammonia forms explosive compounds with gold, MERCURY, or silver. (Eggeman, Tim. mmonia Kirk-Othmer Encyclopedia of Chemical Technology. John Wiley & Sons, Inc. 2001.).Опасность
Central nervous system impairment, peripheral nervous system impairment, and kidney damage. (1) Mercury, metallic: Highly toxic by skin absorption and inhalation of fume or vapor, absorbed by respiratory and intestinal tract. FDA permits zero addition toУгроза здоровью
Mercury is a non-specific toxin, attacking many of the body s systems. At low levels of exposure, symptoms are mainly related to nerve and brain function and include memory loss, mood instability, tremor, and other stress-like symptoms: poor coordination, headache, and visual and hearing problems. Recently, reproductive health has been shown to be affected, with abnormalities in menstrual cycle, poor outcome of pregnancy, and subfertility in both men and women. The immune system is also damaged by mercury exposure.Пожароопасность
Behavior in Fire: Not flammableВоспламеняемость и взрывоопасность
Mercury is not combustible.Профиль безопасности
Poison by inhalation. Human systemic effects by inhalation: wakefulness, muscle weakness, anorexia, headache, tinnitus, hypermotihty, darrhea, liver changes, dermatitis, fever. An experimental teratogen. Experimental reproductive effects. Questionable carcinogen with experimental tumorigenic data. Human mutation data reported. Used in dental applications, electronics, and chemical synthesis. bromopropyne, alkynes + silver perchlorate, ethylene oxide, lithium, methylsilane + oxygen (explodes when shaken), peroxyformic acid, chlorine dioxide, tetracarbonylnickel + oxygen. May react with ammonia to form an explosive product. Mixtures with methyl azide are shockand spark-sensitive explosives. The vapor iptes on contact with boron diiodophosphide. Reacts violently with acetylenic compounds (e.g., acetylene, sodmm acetylide, 2-butyne-l,4 do1 + acid), metals (e.g., aluminum, calcium, potassium, sodium, rubidium, exothermic formation of amalgams), Cl2, ClO2, CH3N3, NazCz, nitromethane. Incompatible with methyl azide, oxidants. When heated to decomposition it emits toxic fumes of Hg. See also MERCURY COMPOUNDS.Возможный контакт
Mercury is used as a catalyst, in dental applications; and in pharmaceuticals; as a liquid cathode in cells for the electrolytic production of caustic and chlorine. It is used in electrical apparatus (lamps, rectifiers, and batteries) and in control instruments (switches, thermometers, and barometers)Канцерогенность
There is no conclusive evidence from epidemiological studies that mercury increases cancer risk in humans.12 In the few studies in which increases have been reported, concomitant exposure to other known carcinogens has confounded the results. The IARC has determined that there is inadequate evidence in humans for the carcinogenicity of mercury and mercury compounds.12 In animals there is inadequate evidence for carcinogenicity of metallic mercury and limited evidence for the carcinogenicity of mercuric chloride.Экологическая судьба
Mercury cycles through various environmental phases by exchange from ground to air and back again. Metallic and dimethylmercury, which are volatile, will be released as mercury vapor that can travel long distances before being redeposited. When found in surface waters and soils it will degas into the surrounding air where natural currents and winds spread the materials until they are deposited back on the surface waters and soils. The majority of mercury returned to the soil or water is by wet partition and accounts for almost all of the mercury found in lakes with no other input source. Inert mercury will deposit bound to particulates in aerosols. Once deposited, mercury must adsorb to soil or sediment particulates or be returned to the atmosphere. This cycle continues with a portion of the mercury revolatilizing into the atmosphere in each cycle.хранилище
Precautions should be taken to prevent spills of mercury because drops of the liquid metal can easily become lodged in floor cracks, behind cabinets, and equipment, etc., with the result that the mercury vapor concentration in the laboratory may then exceed the safe and allowable limits. Containers of mercury should be kept tightly sealed and stored in secondary containers (such as a plastic pan or tray) in a well-ventilated area. When breakage of instruments or apparatus containing significant quantities of Hg is possible, the equipment should be placed in a plastic tray or pan that is large enough to contain the mercury in the event of an accident. Transfers of mercury between containers should be carried out in a fume hood over a tray or pan to confine any spills.Перевозки
UN2809 Mercury, Hazard class: 8; Labels: 8-Corrosive material, 6.1-Poisonous materialМетоды очистки
After air has been bubbled through mercury for several hours to oxidise metallic impurities, it is filtered to remove coarser particles of oxide and dirt, then sprayed through a 4-ft column containing 10% HNO3. It is washed with distilled water, dried with filter paper and distilled under vacuum. [Schenk in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p8 1963.]Несовместимости
Heating mercury causes the formation of toxic mercury oxide fumes. Reacts violently with alkali metals; acetylene, azides, ammonia gas; chlorine, chlorine dioxide; many acids; most metals; ground mixtures of sodium carbide, and ethylene oxide. Contact with methyl azide forms shock- and spark-sensitive explosives. Attacks copper and many other metals, forming amalgamsУтилизация отходов
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. Accumulate for purification and re-use if possible. Mercury vapors may be adsorbed or treated with sulfide solutions and then sent to mercury recovery operationsМеркурий запасные части и сырье
запасной предмет
- OCTACOSANOIC ACID
- Ртуть II хлористая
- 1 5-АНТРАХИНОНДИСУЛЬФОННАЯ КИСЛОТА
- MERCURY(I) ACETATE
- 1-Anthraquinonesulfonic acid
- Ртути йодид
- Реактив Несслера
- Гидроксид калия
- MERCURIC NITRATE, MONOHYDRATE
- 3 - (4-хлорфенил) пропионовой кислоты
- MERCUROUS CHLORIDE
- ФЕНИЛНИТРАТ РТУТИ
- 2-хлор-5-цианопиридин
- MERCUROUS NITRATE
- Аминомеркуриевый хлорид
1of6
Меркурий поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-16632316109 | China | 1090 | 58 | ||
| +86-16632312509 +86-16632315022 |
China | 90 | 58 | ||
| +8619931165850 | China | 1000 | 58 | ||
| +86-19322905052 +86-19322905052 |
China | 9 | 58 | ||
| +8615373025980 | China | 870 | 58 | ||
| +8617756083858 | China | 994 | 58 | ||
| +undefined15030412209 | China | 387 | 58 | ||
| 18871490254 | CHINA | 28180 | 58 | ||
| 18631714998 | CHINA | 906 | 58 | ||
| 86-13657291602 | CHINA | 22968 | 58 |