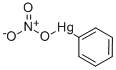
ФЕНИЛНИТРАТ РТУТИ
- английское имяPHENYLMERCURY NITRATE
- CAS №55-68-5
- CBNumberCB3738746
- ФормулаC6H5HgNO3
- мольный вес339.7
- EINECS200-242-9
- номер MDLMFCD00137341
- файл Mol55-68-5.mol
| Температура плавления | 188-190℃ (decomposition) |
| растворимость | Very slightly soluble in water and in ethanol (96 per cent), slightly soluble in hot water. It dissolves in glycerol and in fatty oils. |
| форма | solid |
| Стабильность | Stable. |
| FDA 21 CFR | 310.545 |
| FDA UNII | CG8692ZN14 |
| Код УВД | D09AA04 |
| Система регистрации веществ EPA | Phenylmercuric nitrate (55-68-5) |
| РИДАДР | 1895 |
| Класс опасности | 6.1(a) |
| Группа упаковки | II |
| Банк данных об опасных веществах | 55-68-5(Hazardous Substances Data) |
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)




-
сигнальный язык
опасность
-
вредная бумага
H301:Токсично при проглатывании.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H314:При попадании на кожу и в глаза вызывает химические ожоги.
H410:Чрезвычайно токсично для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P273:Избегать попадания в окружающую среду.
P280:Использовать перчатки/ средства защиты глаз/ лица.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P301+P330+P331:ПРИ ПРОГЛАТЫВАНИИ: Прополоскать рот. Не вызывать рвоту!
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340:ПРИ ВДЫХАНИИ: Свежий воздух, покой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P310:Немедленно обратиться за медицинской помощью.
P314:В случае плохого самочувствия обратиться к врачу.
P363:Перед повторным использованием выстирать загрязненную одежду.
P391:Ликвидировать просыпания/проливы/утечки.
P405:Хранить в недоступном для посторонних месте.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
ФЕНИЛНИТРАТ РТУТИ химические свойства, назначение, производство
Химические свойства
white crystalline powderИспользование
Pharmaceutic aid (antimicrobial agent).Методы производства
Phenylmercuric nitrate is readily formed by heating benzene with mercuric acetate, and treating the resulting acetate with an alkali nitrate.Общее описание
Lustrous scales decomposing at 187-190°C. Very slightly soluble in water. Used as an antiseptic, germicide, fungicide.Реакции воздуха и воды
Very slightly soluble in water.Профиль реактивности
Strongly reactive with many other groups. Incompatible with acids and bases. Organometallics are good reducing agents and therefore incompatible with oxidizing agents. Often reactive with water to generate toxic or flammable gases. Generally highly toxic. Often react on contact with tissues to give toxic products.Угроза здоровью
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.Пожароопасность
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.Фармацевтические приложения
Phenylmercuric salts are used as antimicrobial preservatives mainly in ophthalmic preparations, but are also used in cosmetics, parenteral, and topical pharmaceutical formulations;Phenylmercuric salts are active over a wide pH range against bacteria and fungi and are usually used in neutral to alkaline solutions, although they have also been used effectively at slightly acid pH. In acidic formulations, phenylmercuric nitrate may be preferred to phenylmercuric acetate or phenylmercuric borate as it does not precipitate.
Phenylmercuric nitrate is also an effective spermicide, although its use in vaginal contraceptives is no longer recommended;
A number of adverse reactions to phenylmercuric salts have been reported, and concern at the toxicity of mercury compounds may preclude the use of phenylmercuric salts under certain circumstances;
Профиль безопасности
Poison by intravenous route. FDA over-the-counter drug. When heated to decomposition it emits very toxic fumes of Hg and NOx. See also MERCURY COMPOUNDS and NITRATES.Безопасность
Phenylmercuric nitrate and other phenylmercuric salts have been widely used as antimicrobial preservatives in parenteral and topical pharmaceutical formulations. However, concern over the use of phenylmercuric salts in pharmaceuticals has increased as a result of greater awareness of the toxicity of mercury and other mercury compounds. This concern must, however, be balanced by the effectiveness of these materials as antimicrobial preservatives and the low concentrations in which they are employed.Phenylmercuric salts are irritant to the skin at 0.1% w/w concentration in petrolatum.In solution, they may give rise to erythema and blistering 6–12 hours after administration. In a modified repeated insult patch test, a 2% w/v solution was found to produce extreme sensitization of the skin.
Eye drops containing phenylmercuric nitrate as a preservative should not be used continuously for prolonged periods as mercurialentis, a brown pigmentation of the anterior capsule of the lens may occur. Incidence is 6% in patients using eye drops for greater than 6 years; however, the condition is not associated with visual impairment.Cases of atypical band keratopathy have also been attributed to phenylmercuric nitrate preservative in eye drops.
Concern that the absorption of mercury from the vagina may be harmful has led to the recommendation that phenylmercuric nitrate should not be used in intravaginal formulations.
(mouse, IV): 27 mg/kg
(mouse, oral): 50 mg/kg
(rat, SC): 63 mg/kg
хранилище
All phenylmercuric compound solutions form a black residue of metallic mercury when exposed to light or after prolonged storage. Solutions may be sterilized by autoclaving, although significant amounts of phenylmercuric salts may be lost, hence reducing preservative efficacy, owing to incompatibilities with packaging components or other excipients, e.g. sodium metabisulfite.Phenylmercuric nitrate should be stored in a well-closed container, protected from light, in a cool, dry place.
Несовместимости
The antimicrobial activity of phenylmercuric salts may be reduced in the presence of anionic emulsifying agents and suspending agents, tragacanth, starch, talc, sodium metabisulfite,sodium thiosulfate,disodium edetate,and silicates (bentonite, aluminum magnesium silicate, magnesium trisilicate, and kaolin).Phenylmercuric salts are incompatible with halides, particularly bromides and iodides, as they form less-soluble halogen compounds. At concentrations of 0.002% w/v precipitation may not occur in the presence of chlorides. Phenylmercuric salts are also incompatible with aluminum and other metals, ammonia and ammonium salts, amino acids, and with some sulfur compounds, e.g. in rubber.
Phenylmercuric salts are absorbed by rubber stoppers and some types of plastic packaging components; uptake is usually greatest to natural rubbers and polyethylene, and least to polypropylene. Incompatibilities with some types of filter membranes may also result in loss of phenylmercuric salts following sterilization by filtration.
Регуляторный статус
Included in the FDA Inactive Ingredients Database (parenteral and ophthalmic preparations). Included in parenteral products and eye drops in the EU. Included in the Canadian List of Acceptable Nonmedicinal Ingredients (ophthalmic, nasal and otic preparations only up to 0.002%; there must be no other suitable alternative preservative).Prohibited in first aid antiseptic drug products, antimicrobial diaper rash drug products and vaginal contraceptive drug products in the USA. Limited uses permitted in Japan and the EU for cosmetics.
ФЕНИЛНИТРАТ РТУТИ запасные части и сырье
сырьё
ФЕНИЛНИТРАТ РТУТИ поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия |
|---|---|---|---|---|
| 18871490254 | CHINA | 28172 | 58 | |
| 86-13657291602 | CHINA | 22963 | 58 | |
| +86-023-6139-8061 +86-86-13650506873 |
China | 39894 | 58 | |
| +86-029-89586680 +86-18192503167 |
China | 7724 | 58 | |
| +8618950047208 | China | 43416 | 58 | |
| +86-0592-6210733 | China | 32343 | 55 | |
| 86-21-51086038 | CHINA | 23912 | 58 | |
| 021-021-021-67601398-809-809-809 15221380277 |
China | 9658 | 60 | |
| 0571-+86-571-87396432 | China | 11734 | 59 | |
| 13096311329 028-88469284 616445927 | China | 2874 | 50 |