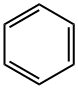
Бензол
- английское имяBenzene
- CAS №71-43-2
- CBNumberCB6854153
- ФормулаC6H6
- мольный вес78.11
- EINECS200-753-7
- номер MDLMFCD00003009
- файл Mol71-43-2.mol
| Температура плавления | 5.5 °C (lit.) |
| Температура кипения | 80 °C (lit.) |
| плотность | 0.874 g/mL at 25 °C (lit.) |
| плотность пара | 2.77 (vs air) |
| давление пара | 166 mm Hg ( 37.7 °C) |
| показатель преломления | n |
| Fp | 12 °F |
| температура хранения | room temp |
| растворимость | Miscible with alcohol, chloroform, dichloromethane, diethyl ether, acetone and acetic acid. |
| пка | 43(at 25℃) |
| форма | Liquid |
| цвет | APHA: ≤10 |
| Относительная полярность | 0.111 |
| Запах | Paint-thinner-like odor detectable at 12 ppm |
| Порог?обнаружения?запаха? | 2.7ppm |
| Пределы взрываемости | 1.4-8.0%(V) |
| Растворимость в воде | 0.18 g/100 mL |
| λмакс | λ: 280 nm Amax: 1.0 λ: 290 nm Amax: 0.15 λ: 300 nm Amax: 0.06 λ: 330 nm Amax: 0.02 λ: 350-400 nm Amax: 0.01 |
| Мерк | 14,1066 |
| БРН | 969212 |
| констант закона Генри | 10.4 at 45.00 °C, 11.4 at 50.00 °C, 13.3 at 55.00 °C, 14.5 at 60.00 °C, 16.8 at 65.00 °C, 19.2 at 70.00 °C (static headspace-GC, Park et al., 2004) |
| Пределы воздействия | TLV-TWA 10 ppm (~32 mg/m3) (ACGIH and OSHA); ceiling 25 ppm (~80 mg/m3) (OSHA and MSHA); peak 50 ppm (~160 mg/m3)/10 min/8 h (OSHA); carcinogenicity: Suspected Human Carcinogen (ACGIH), Human Sufficient Evidence (IARC). |
| Диэлектрическая постоянная | 2.3(20℃) |
| Стабильность | Stable. Substances to be avoided include strong oxidizing agents, sulfuric acid, nitric acid, halogens. Highly flammable. |
| ИнЧИКей | UHOVQNZJYSORNB-UHFFFAOYSA-N |
| Стандарт первичной питьевой воды EPA | MCL:0.005,MCLG:zero |
| LogP | 2.130 |
| Вещества, добавляемые в пищу (ранее EAFUS) | BENZENE |
| FDA 21 CFR | 165.110 |
| Справочник по базе данных CAS | 71-43-2(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 10 |
| Словарь онкологических терминов NCI | benzene |
| FDA UNII | J64922108F |
| Предложение 65 Список | Benzene |
| Справочник по химии NIST | Benzene(71-43-2) |
| МАИР | 1 (Vol. 29, Sup 7. 100F, 120) 2018 |
| Система регистрации веществ EPA | Benzene (71-43-2) |
| UNSPSC Code | 77101502 |
| NACRES | NA.24 |
| Коды опасности | F,T | |||||||||
| Заявления о рисках | 45-46-11-36/38-48/23/24/25-65-39/23/24/25-23/24/25 | |||||||||
| Заявления о безопасности | 53-45-36/37-16-7 | |||||||||
| РИДАДР | UN 1114 3/PG 2 | |||||||||
| OEB | E | |||||||||
| OEL | TWA: 0.1 ppm, STEL: 1 ppm | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | CY1400000 | |||||||||
| F | 3-10 | |||||||||
| Температура самовоспламенения | 560 °C | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 2902 20 00 | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| Банк данных об опасных веществах | 71-43-2(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 orally in young adult rats: 3.8 ml/kg (Kimura) | |||||||||
| ИДЛА | 500 ppm | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H319:При попадании в глаза вызывает выраженное раздражение.
H350:Может вызывать раковые заболевания.
H372:Поражает органы в результате многократного или продолжительного воздействия.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H304:Может быть смертельным при проглатывании и последующем попадании в дыхательные пути.
H340:Может вызывать генетические дефекты.
H412:Вредно для водных организмов с долгосрочными последствиями.
-
оператор предупредительных мер
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P273:Избегать попадания в окружающую среду.
P301+P310:ПРИ ПРОГЛАТЫВАНИИ: Немедленно обратиться за медицинской помощью. Прополоскать рот.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P305+P351+P338:ПРИ ПОПАДАНИИ В ГЛАЗА: Осторожно промыть глаза водой в течение нескольких минут. Снять контактные линзы, если Вы ими пользуетесь и если это легко сделать. Продолжить промывание глаз.
P331:немедленно всю загрязненную одежду. Промыть кожу водой. Не вызывать рвоту!
Бензол химические свойства, назначение, производство
Описание
Benzene is a colorless, volatile, highly flammable liquid that is used extensively in the chemical industry and received wide interest in the early days of organic chemistry.
Because of its structure, benzene is a very stable organic compound. It does not readily undergo addition reactions. Addition reactions involving benzene require high temperature, pressure, and special catalysts. The most common reactions involving benzene involve substitution reactions. Numerous atoms and groups of atoms may replace a hydrogen atom or several hydrogen atoms in benzene. Th ree important types of substitution reactions involving benzene are alkylation, halogenation, and nitration. In alkylation, an alkyl group or groups substitute for hydrogen(s).
Химические свойства
Benzene is a clear, volatile, colorless, highly flammable liquid with a pleasant, characteristic odor. It is an aromatic hydrocarbon that boils at 80.1 DC. Benzene is used as a solvent in many areas of industries, such as rubber and shoe manufacturing, and in the production of other important substances, such as styrene, phenol, and cyclohexane. It is essential in the manufacture of detergents, pesticides, solvents, and paint removers. It is present in fuels such as gasoline up to the level of 5%.Физические свойства
Clear, colorless to light yellow watery liquid with an aromatic, musty, phenolics or gasoline-like odor. At 40 °C, an odor threshold concentration of 190 μg/L in air was determined by Young et al. (1996). An odor threshold of 4.68 ppmv was determined by Leonardos et al. (1969). A detection odor threshold concentration of 108 mg/m3 (34 ppmv) was reported by Punter (1983). The average least detectable odor threshold concentrations in water at 60 °C and in air at 40 °C were 0.072 and 0.5 mg/L, respectively (Alexander et al., 1982).Вхождение
Detectable levels of benzene have been found in a number of soft drinks that contain either a sodium or potassium benzoate preservative and ascorbic acid, and 'diet' type products containing no added sugar are reported to be particularly likely to contain benzene at detectable levels. Surveys carried out in the USA, the UK and Canada have all confirmed that a small proportion of these products may contain low levels of benzene. For example, in a survey of 86 samples analysed by the FDA between April 2006 and March 2007, only five products were found to contain benzene at concentrations above 5 ug kg-1. The levels found were in a range from approximately 10–90 ug kg-1. A survey of 150 UK-produced soft drinks by the Food Standards Agency (FSA) published in 2006 showed that four products contained benzene at levels above 10 ug kg-1, and the highest level recorded was 28 ug kg-1. However, it has been reported that higher levels may develop in these products during prolonged storage, especially if they are exposed to daylight.Benzene may also be formed in some mango and cranberry drinks in the absence of added preservatives, because these fruits contain natural benzoates.
История
Benzene was discovered in 1825 by Michael Faraday (1791–1867), who identified it in a liquid residue from heated whale oil. Faraday called the compound bicarburet of hydrogen, and its name was later changed to benzin by Eilhardt Mitscherlich (1794–1863), who isolated the compound from benzoin (C14H12O2).Использование
Manufacturing of ethylbenzene (for styrene monomer), dodecylbenzene (for detergents), cyclo- hexane (for nylon), phenol, nitrobenzene (for ani- line), maleic anhydride, chlorobenzene, diphenyl, benzene hexachloride, benzene-sulfonic acid, and as a solvent.Определение
ChEBI: Benzene is a six-carbon aromatic annulene in which each carbon atom donates one of its two 2p electrons into a delocalised pi system. A toxic, flammable liquid byproduct of coal distillation, it is used as an industrial solvent. Benzene is a carcinogen that also damages bone marrow and the central nervous system.Методы производства
Today benzene, which is a natural component of petroleum, is obtained from petroleum by several processes. Toluene hydrodealkylation involves mixing toluene (C6H5CH3) and hydrogen in the presence of catalysts and temperatures of approximately 500°C and pressures of about 50 atmospheres to produce benzene and methane: C6H5CH3 + H2 → C6H6 + CH4. Hydrodealkylation strips the methyl group from toluene to produce benzene. Toluene disproportionation involves combining toluene so that the methyl groups bond to one aromatic ring, producing benzene and xylene. Benzene can also be obtained from petroleum reforming in which temperature, pressure, and catalysts are used to convert petroleum components to benzene, which can then be extracted using solvents and distillation processes. Another source of benzene is pyrolysis gasoline or pygas.Реакции
Benzene reacts (1) with chlorine, to form (a) substitution products (one-half of the chlorine forms hydrogen chloride) such as chlorobenzene, C6H5Cl; dichlorobenzene, C6H4Cl2(1,4) and (1,2); trichlorobenzene, C6H3Cl3(1,2,4); tetrachlorobenzene (1,2,3,5); and (b) addition products, such as benzene dichloride C6H6Cl2; benzene tetrachloride, C6H6Cl4; and benzene hexachloride, C6H6Cl6. The formation of substitution products of the benzene nucleus, whether in benzene or its homologues, is favored by the presence of a catalyzer, e.g., iodine, phosphorus, iron; (2) with concentrated HNO3, to form nitrobenzene, C6H5NO2; 1,3- dinitrobenzene, C6H4(NO2)2 (1,3), 1,3,5-trinitrobenzene, C6H3(NO2)3 (1,3,5); (3) with concentrated H2SO4, to form benzene sulfonic acid, C6H5SO3H, benzene disulfonic acid, C6H4(SO3H)2(1,3), benzene trisulfonic acid, C6H3(SO3H)3 (1,3–5); (4) with methyl chloride plus anhydrous aluminum chloride (Friedel-Crafts reaction) to form toluene, monomethyl benzene, C6H5CH3; dimethyl benzene C6H4(CH3)2; trimethyl benzene, C6H3(CH3)3; (5) with acetyl chloride plus anhydrous aluminum chloride (Friedel-Crafts reaction) to form acetophenone (methylphenyl ketone), C6H5COCH3.Общее описание
Benzene appears as a clear colorless liquid with a petroleum-like odor. Flash point less than 0 °F. Less dense than water and slightly soluble in water. Hence floats on water. Vapors are heavier than air.Реакции воздуха и воды
Highly flammable. Slightly soluble in water.Профиль реактивности
Benzene reacts vigorously with allyl chloride or other alkyl halides even at minus 70°C in the presence of ethyl aluminum dichloride or ethyl aluminum sesquichloride. Explosions have been reported [NFPA 491M 1991]. Ignites in contact with powdered chromic anhydride [Mellor 11:235 1946-47]. Incompatible with oxidizing agents such as nitric acid. Mixtures with bromine trifluoride, bromine pentafluoride, iodine pentafluoride, iodine heptafluoride and other interhalogens can ignite upon heating [Bretherick 5th ed. 1995]. Benzene and cyanogen halides yield HCl as a byproduct (Hagedorn, F. H. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.). The reaction of Benzene and trichloroacetonitrile evolves toxic chloroform and HCl gasses. (Hagedorn, F., H.-P. Gelbke, and Federal Republic of Germany. 2002. Nitriles. In Ullman Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA.).Опасность
The acute toxicity of benzene is low. Inhalation of benzene can cause dizziness, euphoria, giddiness, headache, nausea, drowsiness, and weakness. Benzene can cause moderate irritation to skin and severe irritation to eyes and mucous membranes. Benzene readily penetrates the skin to cause the same toxic effects as inhalation or ingestion. The chronic toxicity of benzene is significant. Exposure to benzene affects the blood and blood-forming organs such as the bone marrow, causing irreversible injury; blood disorders including anemia and leukemia may result. The symptoms of chronic benzene exposure may include fatigue, nervousness, irritability, blurred vision, and labored breathing. Benzene is regulated by OSHA as a carcinogen (Standard 1910.1028) and is listed in IARC Group 1 ("carcinogenic to humans"). This substance is classified as a "select carcinogen" under the criteria of the OSHA Laboratory Standard.Воспламеняемость и взрывоопасность
Benzene is a highly flammable liquid (NFPA rating = 3), and its vapors may travel a considerable distance to a source of ignition and "flash back." Vapor-air mixtures are explosive above the flash point. Carbon dioxide and dry chemical extinguishers should be used to fight benzene fires.Химическая реактивность
Reactivity with Water No reaction; Reactivity with Common Materials: No reactions; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.Промышленное использование
Benzene (C6H6, CAS No. 71-43-2) is an aromatic hydrocarbon compound used extensively in the chemical industry as an intermediate in the manufacture of polymers and other products. It is also a common atmospheric contaminant and is present in motor vehicle exhaust emissions and cigarette smoke.In 1990, it was discovered by the USA soft drinks industry that benzene could be produced at low levels in certain soft drinks containing a benzoate preservative and ascorbic acid. Since benzene is a known human carcinogen, its presence in food and beverages is clearly undesirable.
Профиль безопасности
Confirmed human carcinogen producing myeloid leukemia, Hodgkin's dsease, and lymphomas by inhalation. Experimental carcinogenic, neoplastigenic, and tumorigenic data. A human poison by inhalation. An experimental poison by skin contact, intraperitoneal, intravenous, and possibly other routes. Moderately toxic by ingestion and subcutaneous routes. A severe eye and moderate sktn irritant. Human systemic effects by inhalation and ingestion: blood changes, increased body temperature. Experimental teratogenic and reproductive effects. Human mutation data reported. A narcotic. In industry, inhalation is the primary route of chronic benzene poisoning. Poisoning by skin contact has been reported. Recent (1 987) research indicates that effects are seen at less than 1 ppm. Exposures needed to be reduced to 0.1 ppm before no toxic effects were observed. Elimination is chiefly through the lungs.Возможный контакт
Benzene is used as a constituent in motor fuels; as a solvent for fats; inks, oils, paints, plastics, and rubber, in the extraction of oils from seeds and nuts; in photogravure printing. It is also used as a chemical intermediate. By alkylation, chlorination, nitration, and sulfonation, chemicals, such as styrene, phenols, and malefic anhydride are produced. Benzene is also used in the manufacture of detergents, explosives, pharmaceuticals; in the manufacture of cyclohexane and ethylbenzene; and dye-stuffs. Increased concern for benzene as a significant environmental pollutant arises from public exposure to the presence of benzene in gasoline and the increased content in gasoline due to requirements for unleaded fuels for automobiles equipped with catalytic exhaust converters.Канцерогенность
Benzene is known to be a human carcinogen based on sufficient evidence of carcinogenicity from studies in humans.Экологическая судьба
Benzene is released to air primarily by vaporization and combustion emissions associated with its use in gasoline. Other sources are vapors from its production and use in manufacturing other chemicals. In addition, benzene may be in industrial effluents discharged into water and accidental releases from gas and oil production, refining and distribution industries. Benzene released to soil will either evaporate very quickly or leach to groundwater. It can be biodegraded by soil and groundwater microbes. Benzene released to surface water should mostly evaporate within a few hours to a few days, depending on quantity, temperature, water turbulence, etc. Although benzene does not degrade by hydrolysis, it may be biodegraded by microbes.хранилище
work with benzene should be conducted in a fume hood to prevent exposure by inhalation, and splash goggles and impermeable gloves should be worn at all times to prevent eye and skin contact. Benzene should be used only in areas free of ignition sources.Перевозки
UN1114 Benzene, Hazard Class: 3; Labels: 3— Flammable liquidНесовместимости
Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, many fluorides and perchlorates, nitric acid.Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Dilution with alcohol or acetone to minimize smoke is recommended. Bacterial degradation is also possible.Нормативный документ
Current USA and EU legislation does not set maximum limits for benzene in soft drinks. However, the FDA has adopted the Environmental Protection Agency (EPA) maximum contaminant level (MCL) for drinking water of 5 ppb as a quality standard for bottled water. ThisMCL has been used to evaluate the significance of benzene contamination in the soft drinks tested in surveys. The FSA has used the World Health Organization (WHO) guideline level for benzene in water of 10 mg kg-1 as a point of reference for its own survey results.Бензол запасные части и сырье
сырьё
1of3
запасной предмет
- 1-АЗИРИДИНЭТАНОЛ
- ЭТИЛОВЫЙ ЭФИР 5-ТРЕТ-БУТИЛ-1H-ИНДОЛ-2-КАРБОНОВОЙ КИСЛОТЫ
- Nonanophenone
- tert-Amylbenzene
- 4-Benzoylbutyric кислота
- Изобутирофенона
- Этил изоникотиноилацета
- 2-метоксифенилизоциана
- Acetrizoic acid
- Aminoethanolcarboxylate heptadecyl carbonate
- 4-(2-тиенил)бензальдегид
- 2 - (гептаноил) тиофен
- 2-хлорфенил изоцианатом
- Butyl 3-mercaptopropionate
- ФЕНИЛНИТРАТ РТУТИ
- 4-Бром-7-хлорхинолин
- 2,5-диметил-3-гексин-2 ,5-диол
- 4-метоксифенил изоциана
- 2-фторфенилизоциана
- Benzoylcyclohexane
- ISOCYANIC ACID
- Изомераза, глюкоза
- Sulfachloropyridazine sodium
- 3-Нитробензоилхлорид
- Pesticide synergist agent
- 1,3-ДИМЕТИЛ-2-(2-ТИЕНИЛ)ИМИДАЗОЛИДИН
- 3-бромпропионитрил
- Octyl cyanoacetate
- Дихлорфенилборан
- 4-фторфенил изоциана
1of8
Бензол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| 19565631292 19565631292 |
China | 270 | 58 | ||
| +1-3026880818 +1-3026880818 |
Canada | 66 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +undefined-21-51877795 | China | 32965 | 60 | ||
| +86-13734021967 +8613734021967 |
China | 1001 | 58 | ||
| 13867897135 | CHINA | 923 | 58 | ||
| 18871490254 | CHINA | 28172 | 58 | ||
| 86-13657291602 | CHINA | 22963 | 58 | ||
| 18853181302 | CHINA | 5906 | 58 |