
Диэтиленгликоля
- английское имяDiethylene glycol
- CAS №111-46-6
- CBNumberCB0409579
- ФормулаC4H10O3
- мольный вес106.12
- EINECS203-872-2
- номер MDLMFCD00002882
- файл Mol111-46-6.mol
химическое свойство
| Температура плавления | −10 °C(lit.) |
| Температура кипения | 245 °C(lit.) |
| плотность | 1.118 g/mL at 25 °C(lit.) |
| плотность пара | 2.14 (vs air) |
| давление пара | 0.01 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 143 °C |
| температура хранения | Keep in dark place,Sealed in dry,Room Temperature |
| растворимость | H2O: 50 mg/mL at 20 °C, clear, colorless |
| пка | 14.03±0.10(Predicted) |
| форма | Oily Liquid |
| цвет | colorless |
| Относительная полярность | 0.713 |
| РН | 5.5-7.0 (25℃, 50mg/mL in H2O) |
| Запах | Practically odorless. |
| Пределы взрываемости | 2-12.3% |
| Растворимость в воде | SOLUBLE |
| Точка замерзания | -10.45℃ |
| Чувствительный | Hygroscopic |
| λмакс | λ: 260 nm Amax: ≤0.02 λ: 280 nm Amax: ≤0.01 |
| Мерк | 14,3119 |
| БРН | 969209 |
| Диэлектрическая постоянная | 31.7(20℃) |
| Стабильность | Hygroscopic |
| ИнЧИКей | MTHSVFCYNBDYFN-UHFFFAOYSA-N |
| LogP | -1.98 at 20℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | DIETHYLENE GLYCOL |
| FDA 21 CFR | 175.105; 175.300; 175.320; 177.2420; 177.2800 |
| Справочник по базе данных CAS | 111-46-6(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2-5 |
| FDA UNII | 61BR964293 |
| Справочник по химии NIST | Ethanol, 2,2'-oxybis-(111-46-6) |
| Система регистрации веществ EPA | Diethylene glycol (111-46-6) |
| UNSPSC Code | 41116107 |
| NACRES | NA.25 |
больше
| Коды опасности | Xn,T,Xi | |||||||||
| Заявления о рисках | 22 | |||||||||
| Заявления о безопасности | 46 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | ID5950000 | |||||||||
| F | 10 | |||||||||
| Температура самовоспламенения | 442 °F | |||||||||
| Примечание об опасности | Toxic/Irritant | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29094100 | |||||||||
| Банк данных об опасных веществах | 111-46-6(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in rats, guinea pigs (g/kg): 20.76, 13.21 orally (Smyth) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)

-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
-
оператор предупредительных мер
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Диэтиленгликоля химические свойства, назначение, производство
Химические свойства
Diethylene glycol is a clear colorless, odorless and stable oily liquid. It is also slightly viscous, noncorrosive and nonvolatile. Because of its ether and alcohol group, diethylene glycol exhibits chemical properties characteristic of both primary alcohols and ethers. Its boiling point is considerably higher than that of ethylene glycol, and its solvent is greater. Diethylene glycol is miscible with water, ethers, lower aliphatic alcohols, aldehydes and ketones and is partially soluble in benzene, carbon tetrachloride, monobenzene, orthodichlorobenzene and toluene. It dissolves many dyes, resins, oils, nitrocellulose and many organic substances. Because of its solvent power, low volatility and hygroscopicity, it is used in textile lubricants, cutting oils, dry cleaning soap, printing inks, steam-set inks, and nongrain wood stains. In the textile industry diethylene glycol is used as a conditioning agent for wool, rayon, and cotton. As a solvent for dyes it makes a valuable assistant in dyeing and printing. The high hygroscopicity of diethylene glycol makes it an efficient softening agent for tobacco, paper, synthetic sponges, glues and casein. Diethylene glycol is especially useful in the dehydration of natural gas. A mixture of diethylene glycol and monoethanolamine will remove moisture, hydrogen sulfide and carbon dioxide from natural gas.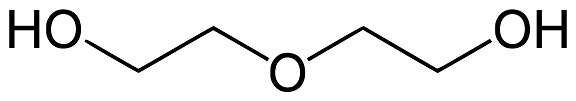
diethylene glycol structure
Методы производства
Diethylene glycol is produced commercially as a by-product of ethylene glycol production. It can also be produced directly by reaction between ethylene glycol and ethylene oxide .прикладной
Diethylene glycol has many industrial uses. It is a component of antifreeze, brake fluids, cosmetics, inks, and drying agents, and it is used as a plasticizer. In antifreeze solution for sprinkler systems, water seals for gas tanks, etc. (water with 40% diethylene glycol freezes at -18°; with 50% at -28°); as lubricating and finishing agent for wool, worsted, cotton, rayon, and silk; as solvent for vat dyes; in composition corks, glues, gelatin, casein, and pastes to prevent drying out.Общее описание
Diethylene glycol appears as a colorless liquid. Denser than water. Contact may slightly irritate skin, eyes and mucous membranes. May be slightly toxic by ingestion. Used to make other chemicals.Реакции воздуха и воды
Slightly soluble in water.Профиль реактивности
Diethylene glycol is incompatible with strong oxidizing agents. Diethylene glycol is also incompatible with strong bases. Diethylene glycol can react with sulfuric acid and other dehydrating agents, nitric acid, oxygen, hydrogen peroxide, perchloric acid and strong acids. Mixtures with sodium hydroxide decompose exothermically when heated to 446° F.Угроза здоровью
Ingestion of large amounts may cause degeneration of kidney and liver and cause death. Liquid may cause slight skin irritation.Пожароопасность
Diethylene glycol is combustible.Токсикология
The toxicity of diethylene glycol is similar to ethylene glycol and clearly is a CNS depressant. It has a low inhalation hazard because of its low vapor pressure; however, inhalation of the mist or aerosol is to be avoided. Workplace levels for vapors and aerosols cannot exceed 50 ppm. In case of accidental release of diethylene glycol, use of a full-face positive air pressure respirator is recommended. Even though the toxicokinetics in humans is not completely understood, its toxic nature is confirmed by animal studies. Several human cases were reported in the medical literature. Several children in Haiti died in 1995 and 1996 following the consumption of medication containing diethylene glycol. Similar other cases in children were reported in other countries as well. A 24-year-old man developed encephalopathy and rapidly became quadriplegic following ingestion of a solution containing diethylene glycol . Thus, the toxicity of diethylene glycol is well established.Профиль безопасности
Moderately toxic to humans by ingestion. Poison experimentally by inhalation. Moderately toxic by ingestion and intravenous routes. Questionable carcinogen with experimental carcinogenic,tumorigenic, and teratogenic data. An eye and human skin irritant. Combustible when exposed to heat or flame; can react with oxidning materials. To fight fire, use alcohol foam, water, Con, dry chemical. Mixtures with sodium hydroxide decompose exothermically when heated to 230℃ and release explosive hydrogen gas. When heated to decomposition it emits acrid smoke and irritating fumes. See also GLYCOL ETHERS.Канцерогенность
Weil et al. , in their longterm studies on rats of three different age levels, found only one bladder tumor in those fed diets that contained 4% diethylene glycol. This tumor was in a rat that also had bladder stones . To clarify the question of the cause of the tumor, Weil et al. implanted calcium oxalate stones or glass beads into the bladders of rats. They found that bladder tumors never developed without the presence of a foreign body in the bladder. This led to the conclusion that diethylene glycol essentially free of ethylene glycol is not a primary carcinogen.Экологическая судьба
Diethylene glycol is metabolized by alcohol dehydrogenase to toxic metabolites predominantly, HEAA and DGA. DEG can cause an anion gap metabolic acidosis, cortical necrosis resulting in permanent renal failure and neurotoxicity. DGA, not HEAA, was recently identified as being the primary nephrotoxic agent causing proximal tubule cell death. The neurotoxicity seen after DEG poisoning is only recently described. The neurotoxicity is delayed and has cranial and peripheral demyelinating sensorimotor polyneuropathy pattern. The exact mechanism of the neurotoxicity remains unclear and in the cases described in the literature, it appears to be prolonged but does show evidence of reversibility.Диэтиленгликоля запасные части и сырье
сырьё
запасной предмет
- Ксилолы
- polyurethane water-based emulsion finishes PU-II series
- 2,6-Dichloroindophenol sodium salt
- 2-Chloro-4-dodecylphenol
- 2,2'-OXYDIACETYL CHLORIDE
- 4-(2-THIENYL)BUTYRIC ACID
- 1-пиренмасляная кислота
- 4-метилморфолин
- Урсодезоксихолевая кислота
- 4-Амино-2 ,6-дихлорфенола
- Unsaturated polyester resin
- 2-(2-HEXYLOXYETHOXY)ETHANOL
- polyurethae finishes PUC series
- CSF series modified sacrylic binder
- 21-Iodo-16-methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
- AC anti-fungus leather finishing agent
- Толуол
- 2-этоксиэтиловый эфир
- 4 - (4-метоксифенил) масл ной кислоты
- Diethylene glycol dibenzoate
- ЭФИР моноэтиловый диэтиленгликоля
- 6-фенилгексановой кислота
- диамфенетид
- thickening agent PAS
- 1-Хлор-3-фтор-2-пропанол
- Бензол
- 16-Methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
- defoaming agent OTD
- 2,3-DIHYDROXYQUINOXALINE-6-CARBOXYLIC ACID
- Этилен гликол
1of8
Диэтиленгликоля поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0510-87501824 +86-13961572207 |
China | 132 | 58 | ||
| +1-+1(833)-552-7181 | United States | 57505 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-13131129325 | China | 5887 | 58 | ||
| +86-371-86557731 +86-13613820652 |
China | 20259 | 58 | ||
| +86-18400010335 +86-18034520335 |
China | 1015 | 58 | ||
| +8613343047651 | China | 3692 | 58 | ||
| +86-86-17798073498 +8617798073498 |
China | 299 | 58 | ||
| +86-18532138899 +86-18532138899 |
China | 939 | 58 |