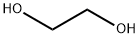
Этилен гликол
- английское имяEthylene glycol
- CAS №107-21-1
- CBNumberCB7852707
- ФормулаC2H6O2
- мольный вес62.07
- EINECS203-473-3
- номер MDLMFCD00002885
- файл Mol107-21-1.mol
| Температура плавления | -13 °C (lit.) |
| Температура кипения | 195-198 °C |
| плотность | 1.113 g/mL at 25 °C (lit.) |
| плотность пара | 2.1 (vs air) |
| давление пара | 0.08 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 230 °F |
| температура хранения | 2-8°C |
| растворимость | water: miscible |
| форма | Viscous Liquid |
| пка | 14.22(at 25℃) |
| цвет | blue |
| Относительная полярность | 0.79 |
| РН | 6-7.5 (100g/l, H2O, 20℃) |
| Запах | Odorless |
| Пределы взрываемости | 3.2%(V) |
| Скорость испарения | 1 |
| Растворимость в воде | miscible |
| Точка замерзания | -11.5℃ |
| Чувствительный | Hygroscopic |
| λмакс | λ: 260 nm Amax: ≤0.03 λ: 280 nm Amax: ≤0.01 |
| Мерк | 14,3798 |
| БРН | 505945 |
| Пределы воздействия | Ceiling limit in air for vapor and mist 50 ppm (~125 mg/m3) (ACGIH); TWA 10 mg/m3 (particulates) (MSHA). |
| Диэлектрическая постоянная | 37.0(20℃) |
| LogP | -1.36 at 25℃ |
| Непрямые добавки, используемые в веществах, контактирующих с пищевыми продуктами | ETHYLENE GLYCOL |
| FDA 21 CFR | 175.105; 175.300; 175.320; 176.300; 177.1680 |
| Справочник по базе данных CAS | 107-21-1(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 3-4 |
| FDA UNII | FC72KVT52F |
| Предложение 65 Список | Ethylene glycol (ingested) |
| Справочник по химии NIST | 1,2-Ethanediol(107-21-1) |
| Система регистрации веществ EPA | Ethylene glycol (107-21-1) |
| UNSPSC Code | 41116121 |
| NACRES | NA.32 |
| Коды опасности | Xn | |||||||||
| Заявления о рисках | 22-36-41 | |||||||||
| Заявления о безопасности | 26-39-36/37/39 | |||||||||
| WGK Германия | 3 | |||||||||
| RTECS | KW2975000 | |||||||||
| Температура самовоспламенения | 752 °F | |||||||||
| TSCA | Yes | |||||||||
| кода HS | 29053100 | |||||||||
| Банк данных об опасных веществах | 107-21-1(Hazardous Substances Data) | |||||||||
| Токсичность | LD50 in rats, guinea pigs (g/kg): 8.54, 6.61 orally (Smyth); in mice (ml/kg): 13.79 orally (Bornmann) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)


-
сигнальный язык
предупреждение
-
вредная бумага
H302:Вредно при проглатывании.
H373:Может поражать органы (Нервная система) в результате многократного или продолжительного воздействия при вдыхании.
-
оператор предупредительных мер
P260:Не вдыхать газ/ пары/ пыль/ аэрозоли/ дым/ туман.
P264:После работы тщательно вымыть кожу.
P270:При использовании продукции не курить, не пить, не принимать пищу.
P301+P312:ПРИ ПРОГЛАТЫВАНИИ: Обратиться за медицинской помощью при плохом самочувствии.
P314:В случае плохого самочувствия обратиться к врачу.
P501:Удалить содержимое/ контейнер на утвержденных станциях утилизации отходов.
Этилен гликол химические свойства, назначение, производство
Описание
Ethylene glycol was first synthesized in 1859; however, it did not become a public health concern until after World War II. In fact, the first published series of deaths from ethylene glycol consumption involved 18 soldiers who drank antifreeze as a substitute for ethanol. Despite the early recognition that patients who drank ethanol in addition to ethylene glycol had prolonged survival when compared to those drinking ethylene glycol alone, antidotal treatment of ethylene glycol toxicity with ethanol was not evaluated until the 1960s. Today, ethylene glycol poisoning continues to be a public health problem, particularly in the southeastern United States. In 2009, US poison control centers received 5282 calls about possible ethylene glycol exposures, and the toxicology community believes these exposures are underreported.Химические свойства
Ethylene glycol,CH20HCH20H, also known as glycol,ethylene alcohol, glycol alcohol, and dihydric alcohol, is a colorless liquid. It is soluble in water and in alcohol. Ethyleneglycol has a low freezing point,-25°C (-13 OF), and is widely used as an antifreeze in automobiles and in hydraulic fluids. It is used as a solvent for nitrocellulose and in the manufacture of acrylonitrile, dynamites, and resins.Использование
Ethylene glycol is used as an antifreeze inheating and cooling systems (e.g., automobileradiators and coolant for airplane motors).It is also used in the hydraulic brake fluids;as a solvent for paints, plastics, and inks; as a softening agent for cellophane; and in themanufacture of plasticizers, elastomers, alkydresins, and synthetic fibers and waxes.Методы производства
Historically, ethylene glycol has been manufactured by hydrolyzing ethylene oxide. Presently, it is also produced commercially by oxidizing ethylene in the presence of acetic acid to form ethylene diacetate, which is hydrolyzed to the glycol, and acetic acid is recycled in the process .Определение
ChEBI: A 1,2-glycol compound produced via reaction of ethylene oxide with water.Подготовка
Ethylene glycol is prepared by the hydration of ethylene oxide:
This reaction is carried out in a manner comparable to that described for the preparation of propylene glycol from propylene oxide . Ethylene glycol is a colourless liquid, b.p. 197??C.
Реакции
Glycol reacts (1) with sodium to form sodium glycol, CH2OH · CH2ONa, and disodium glycol, CH2ONa·CH2ONa; (2) with phosphorus pentachloride to form ethylene dichloride, CH2Cl·CH2Cl (3) with carboxy acids to form mono- and disubstituted esters, e.g., glycol monoacetate, CH2OH·CH2OOCCH3, glycol diacetate, CH3COOCH2 · CH2OOCCH3; (4) with nitric acid (with sulfuric acid), to form glycol mononitrate, CH2OH·CH2ONO2, glycol dinitrate, CH2ONO2 · CH2ONO2; (5) with hydrogen chloride, heated, to form glycol chlorohydrin (ethylene chlorohydrin, CH2OH·CHCl); (6) upon regulated oxidation to form glycollic aldehyde, CH2OH·CHO, glyoxal, CHO · CHO, glycollic acid, CH2OH·COOH, glyoxalic acid, CHO·COOH, oxalic acid, COOH·COOH.Общее описание
Ethylene glycol is a clear, colorless syrupy liquid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Since Ethylene glycol is a liquid Ethylene glycol can easily penetrate the soil and contaminate groundwater and nearby streams.Профиль реактивности
Mixing Ethylene glycol in equal molar portions with any of the following substances in a closed container caused the temperature and pressure to increase: chlorosulfonic acid, oleum, sulfuric acid, [NFPA 1991].Опасность
Questionable carcinogen. Toxic by ingestion and inhalation. Lethal dose reported to be 100 cc.Угроза здоровью
Inhalation of vapor is not hazardous. Ingestion causes stupor or coma, sometimes leading to fatal kidney injury.Пожароопасность
Ethylene glycol is combustible.Профиль безопасности
Human poison by ingestion. (Lethal dose for humans reported to be 100 mL.) Moderately toxic to humans by an unspecified route. Moderately toxic experimentally by ingestion, subcutaneous, intravenous, and intramuscular routes. Human systemic effects by ingestion and inhalation: eye lachrymation, general anesthesia, headache, cough, respiratory stimulation, nausea or vomiting, pulmonary, kidney, and liver changes. If ingested it causes initial central nervous system stimulation followed by depression. Later, it causes potentially lethal kidney damage. Very toxic in particulate form upon inhalation. An experimental teratogen. Other experimental reproductive effects. Human mutation data reported. A skin, eye, and mucous membrane irritant. Combustible when exposed to heat or flame; can react vigorously with oxidants. Moderate explosion hazard when exposed to flame. Iptes on contact with chromium trioxide, potassium permanganate, and sodium peroxide. Mixtures with ammonium dichromate, silver chlorate, sodium chlorite, and uranyl nitrate ipte when heated to 100°C. Can react violently with chlorosulfonic acid, oleum, H2SO4, HClO4, and Pass. Aqueous solutions may ignite silvered copper wires that have an applied D.C. voltage. To fight fire, use alcohol foam, water, foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes.Возможный контакт
Ethylene glycol is used in antifreeze (especially as car radiator antifreeze) and in production of polyethylene terephthalate fibers and films; in hydraulic fluids; antifreeze and coolant mixtures for motor vehicles; electrolytic condensers; and heat exchangers. It is also used as a solvent and as a chemical intermediate for ethylene glycol dinitrate, glycol esters; resins, and for pharmaceuticals.Экологическая судьба
Ethylene glycol is considered an inert ingredient in pesticides. It typically enters the environment through waste streams after use of deicing products, where it is highly mobile in soil and contaminates groundwater. Ethylene glycol is considered ‘readily biodegradable.’ It biodegrades relatively quickly; its half-life (t1/2) is 2–12 days in soil.Ethylene glycol is biodegraded in water under both aerobic and anaerobic conditions within a day to a few weeks. In the atmosphere, ethylene glycol photochemically degrades with a t1/2 of approximately 2 days.
Растворимость в органика
Miscible with water and alcohol, soluble in lower atifatic alcohols and ketones, Propylene glycol and Glycerin, poorly soluble in Hydrocarbons such as Terpenes as well as in Terpene alcohols, esters, etc.Перевозки
UN3082 Environmentally hazardous substances, liquid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name RequiredМетоды очистки
It is very hygroscopic, and also likely to contain higher diols. Dry it with CaO, CaSO4, MgSO4 or NaOH and distil it under vacuum. Dry further by reaction with sodium under nitrogen, reflux for several hours and distil. The distillate is then passed through a column of Linde type 4A molecular sieves and finally distil under nitrogen, from more molecular sieves. Then fractionally distil it. [Beilstein 1 IV 2369.]Несовместимости
Reacts with sulfuric acid, oleum, chlorosulfonic acid; strong oxidizing agents; strong bases; chromium trioxide; potassium permanganate; sodium peroxide. Hygroscopic (i.e., absorbs moisture from the air)Утилизация отходов
Dissolve or mix the material with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber. All federal, state, and local environmental regulations must be observed. Alternatively, ethylene glycol can be recovered from polyester plant wastesЭтилен гликол запасные части и сырье
сырьё
1of2
запасной предмет
- 2,4-DIAMINO-6-MERCAPTOPYRIMIDINE
- 2 - (2-бромэтил) -1,3-диоксолан
- tussah silk fabric aftertreatment finishing agent
- 3-аминофталгидразид
- Щавелевая кислота дигидрат чда
- 3-BENZOYL PROPIOPHENONE
- Dye-fixing agent,no formaldehyde
- solid alcohol
- N-Винил-2-пирролидон
- 6-Azathymine
- 21-Iodo-16-methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
- Ethylene glycol diglycidyl ether
- 1-CHLORO-2-METHYL-1-PROPENE
- 2,3-THIOPHENEDICARBOXALDEHYDE
- 4-(Hydroxymethyl)phenylboronic acid
- Ethylene brassylate
- 2-(2,4-DINITROPHENOXY)ETHANOL
- Antifreeze
- 16,17-Эпоксипрегна-5,9(11)-диен-3,20-дион циклический бис(1,2-этандиилацеталь)
- Saponified soluble oil
- 16-Methylpregna-1,4,9(11)-trien-17-ol-3,20-dione
- 9-Bromo-11,17,21-trihydroxy-16-methylpregna-1,4-diene-3,20-dione-21-acetate
- 16-Methylpregna-4,9(11)-dien-17-ol-3,20-dione
- 17-Ethinyl-17-hydroxy-18-methylestra-5(10),9(11)-dien-3-one-3-ethylene ketal
- 17,21-dihydroxy-16beta-methylpregna-1,4,9(11)-triene-3,20-dione 21-acetate
1of8
Этилен гликол поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-0531-8875-2665 +8613153039501 |
China | 662 | 58 | ||
| +8617653113209 | China | 3049 | 58 | ||
| +86-85511178; +86-85511178; |
China | 35425 | 58 | ||
| +86-86-02137122233 +8613795318958 |
China | 299 | 55 | ||
| +86-17736087130 +86-18633844644 |
China | 994 | 58 | ||
| +1-+1(833)-552-7181 | United States | 57505 | 58 | ||
| +86-15662691337 +86-15662695772 |
China | 998 | 58 | ||
| +8613817748580 | China | 40066 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 |