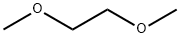
1,2-диметоксиэтан
- английское имя1,2-Dimethoxyethane
- CAS №110-71-4
- CBNumberCB9232185
- ФормулаC4H10O2
- мольный вес90.12
- EINECS203-794-9
- номер MDLMFCD00008502
- файл Mol110-71-4.mol
| Температура плавления | -69 °C |
| Температура кипения | 85 °C(lit.) |
| плотность | 0.867 g/mL at 25 °C(lit.) |
| плотность пара | 3.1 (20 °C, vs air) |
| давление пара | 48 mm Hg ( 20 °C) |
| показатель преломления | n |
| Fp | 32 °F |
| температура хранения | Store below +30°C. |
| растворимость | 1000g/l soluble |
| форма | Liquid |
| цвет | Clear |
| Запах | water-wh. liq., sharp ethereal odor |
| РН | 7 |
| Пределы взрываемости | 10.4% |
| Растворимость в воде | Miscible |
| Чувствительный | Air Sensitive |
| λмакс | λ: 220 nm Amax: 1.00 λ: 250 nm Amax: 0.20 λ: 300 nm Amax: 0.03 λ: 350-400 nm Amax: 0.01 |
| Мерк | 14,3224 |
| БРН | 1209237 |
| Диэлектрическая постоянная | 7.2(25℃) |
| Стабильность | Stable, but explosive peroxides may form on exposure to air. Test for the presence of peroxides before heating. Avoid heat, light, air. Highly flammable. Store under inert gas. |
| ИнЧИКей | XTHFKEDIFFGKHM-UHFFFAOYSA-N |
| LogP | -0.21 at 25℃ |
| Справочник по базе данных CAS | 110-71-4(CAS DataBase Reference) |
| Рейтинг продуктов питания EWG | 2 |
| FDA UNII | GXS24JF5IW |
| Справочник по химии NIST | Ethane, 1,2-dimethoxy-(110-71-4) |
| Система регистрации веществ EPA | Ethylene glycol dimethyl ether (110-71-4) |
| UNSPSC Code | 41116107 |
| NACRES | NA.24 |
| Коды опасности | F,T | |||||||||
| Заявления о рисках | 60-61-11-19-20-38 | |||||||||
| Заявления о безопасности | 53-45 | |||||||||
| РИДАДР | UN 2252 3/PG 2 | |||||||||
| WGK Германия | 1 | |||||||||
| RTECS | KI1451000 | |||||||||
| F | 3-10-23 | |||||||||
| Температура самовоспламенения | 396 °F | |||||||||
| TSCA | Yes | |||||||||
| Класс опасности | 3 | |||||||||
| Группа упаковки | II | |||||||||
| кода HS | 29091900 | |||||||||
| Банк данных об опасных веществах | 110-71-4(Hazardous Substances Data) | |||||||||
| NFPA 704: |
|
рисовальное письмо(GHS)
-
рисовальное письмо(GHS)



-
сигнальный язык
опасность
-
вредная бумага
H315:При попадании на кожу вызывает раздражение.
H225:Легковоспламеняющаяся жидкость. Пары образуют с воздухом взрывоопасные смеси.
H332:Вредно при вдыхании.
H360FD:Может отрицательно повлиять на способность к деторождению. Может отрицательно повлиять на неродившегося ребенка.
-
оператор предупредительных мер
P202:Перед использованием ознакомиться с инструкциями по технике безопасности.
P210:Беречь от тепла, горячих поверхностей, искр, открытого огня и других источников воспламенения. Не курить.
P233:Держать в плотно закрытой/герметичной таре.
P303+P361+P353:ПРИ ПОПАДАНИИ НА КОЖУ (или волосы): Снять/удалить немедленно всю загрязненную одежду. Промыть кожу водой.
P304+P340+P312:ПРИ ВДЫХАНИИ: Свежий воздух, покой. Обратиться за медицинской помощью при плохом самочувствии.
P308+P313:ПРИ подозрении на возможность воздействия обратиться за медицинской помощью.
1,2-диметоксиэтан химические свойства, назначение, производство
Описание
1,2-Dimethoxyethane (DME) is a liquid ether used as an aprotic solvent. It is also known as glyme, monoglyme, dimethyl glycol, ethylene glycol, dimethyl ether, dimethyl cellosolve, and DME. It has the special ability to form chelates thanks to the high flexibility of its chains. Moreover, DME is the molecular model system for the poly(oxyethylene) chain. It is commonly used as a solvent in batteries and in organometallic reaction chemistry since it has higher boiling than diethyl ether and THF[1].Химические свойства
Ethylene glycol dimethyl ether, also called 1,2-Dimethoxyethane, is a colorless liquid with a strong smell similar to ether. It is stable and does not easily react. It can dissolve different resins and cellulose, and can be mixed with water and various organic solvents like alcohols, ketones, and esters in any amount. Its solubility can be changed by diluting it with water or specific solvents.Определение
ChEBI: 1,2-dimethoxyethane is a diether that is the 1,2-dimethyl ether of ethane-1,2-diol. It has a role as a non-polar solvent. It is functionally related to an ethylene glycol.прикладной
Monoglyme serves as an electrolyte solution component for lithium batteries. It is also frequently utilized as a solvent in organometallic reactions, particularly those involving organolithium compounds. Additionally, it can act as a ligand in certain chemical reactions.Общее описание
A liquid with a sharp odor. Less dense than water. Flash point 34°F. Mildly toxic by ingestion and inhalation. Severely irritates skin and eyes. Vapors heavier than air. Used to make other chemicals.Реакции воздуха и воды
Highly flammable. Slightly soluble in water.Профиль реактивности
When the solvent, 1,2-Dimethoxyethane, was poured into a funnel previously used to introduce the lithium aluminum hydride, a fire ignited the funnel, [MCA Case History 1182(1966)].Опасность
Moderate fire risk.Угроза здоровью
If ingested causes nausea, vomiting, cramps, weakness, coma.Пожароопасность
Behavior in Fire: Containers may explode in fires.Профиль безопасности
An experimental teratogen. Other experimental reproductive effects. Readdy forms an explosive peroxide. A very dangerous fire hazard when exposed to heat, flame, or oxidzers. Mixture with lithium tetrahydroaluminate may ignite orexplode if heated. When heated to decomposition it emits acrid smoke and fumes. See also GLYCOL ETHERS.Синтез
1,2-Dimethoxyethane is derived from the reaction of ethylene glycol monomethyl ether with sodium metal and methyl chloride. The ethylene glycol monomethyl ether and the metal sodium were refluxed together until the metal sodium was completely reacted, the temperature was lowered to 45° C., and methyl chloride was introduced. After the reaction is completed, fractional distillation is performed to collect fractions at 84-85.5°C to obtain 1,2-Dimethoxyethane.Методы очистки
Traces of water and acidic materials have been removed from it by refluxing with Na, K or CaH2, decanting and distilling from Na, K, CaH2 or LiAlH4. The reaction has been speeded up by using vigorous high-speed stirring with molten potassium. For virtually complete elimination of water, 1,2-dimethoxyethane has been dried with Na-K alloy until a characteristic blue colour is formed in the solvent at Dry-ice/cellosolve temperatures: the solvent is kept with the alloy until distilled for use [Ward J Am Chem Soc 83 1296 1961]. Alternatively, glyme, refluxed with benzophenone and Na-K, is dry enough if, on distillation, it gives a blue colour of the ketyl immediately on addition to benzophenone and sodium [Ayscough & Wilson J Chem Soc 5412 1963]. It has also been purified by distillation under N2 from sodium benzophenone ketyl (see above). [Beilstein 1 IV 2376.]1,2-диметоксиэтан запасные части и сырье
сырьё
запасной предмет
- Tetrahydrocurcumin
- 2-Aminoquinoline
- 3-аминобифенил
- 5-CHLORO-2'-DEOXYURIDINE
- Трет-бутил 4-(4-формил-1,3-тиазол-2-ил)тетрагидро-1(2H)-пиридинкарбоксила
- 2-Bromo-5-hydroxypyridine radical ion(1+)
- 4-BENZYL-MORPHOLINE-2-CARBOXYLIC ACID
- 1-METHYL-1H-INDOLE-5-BORONIC ACID 2,2-DIMETHYL PROPANE DIOL-1,3-CYCLIC ESTER
- EUROPIUM D-3-TRIFLUOROACETYLCAMPHORATE
- 2-Chloro-5-cyano-3-methylpyridine
- Quinacridone
- 4-хлор-1,3-диметил-1H-пиразолo[3,4-b]пиридин-5-карбоновая кислота
- 3-N-Boc-amino-azetidine
- 4-CHLORO-7-METHYLTHIENO[3,2-D]PYRIMIDINE
- 3-ТРИФТОРАЦЕТИЛ-D-КАМФОРА
- 2-CHLORO-5-IODO-3-METHYLPYRIDINE
- 7-Methylthieno[3,2-d]pyrimidin-4(3H)-one
- ЭТИЛ-4-МЕТОКСИ-3-ОКСОБУТАНОАТ
- Сарафлоксацин
- 10-Undecynoic кислота
- 3,4-Дихлор-N-метиланилина
- N,N'-Dimethylquinacridone
- THIOPHENE-2,5-DICARBOXYLIC ACID MONOMETHYL ESTER
- 4-(Dicyanomethylene)-2-tert-butyl-6-(1,1,7,7-tetramethyljulolidin-4-yl-vinyl)-4H-pyran
- TERT-BUTYL 4-(5-FORMYL-4-METHYL-1,3-THIAZOL-2-YL)PIPERIDINE-1-CARBOXYLATE
1of8
1,2-диметоксиэтан поставщик
| поставщик | телефон | страна | номенклатура продукции | благоприятные условия | |
|---|---|---|---|---|---|
| +86-5638152626 +86-17756305689 |
China | 10 | 58 | ||
| +8613817748580 | China | 40066 | 58 | ||
| +8615531157085 | China | 8804 | 58 | ||
| +86 13288715578 +8613288715578 |
China | 12825 | 58 | ||
| +86-(0)57185586718 +86-13336195806 |
China | 29792 | 60 | ||
| +86-0371-55170693 +86-19937530512 |
China | 21632 | 55 | ||
| +86-0551-65418679 +8618949832763 |
China | 2986 | 55 | ||
| +86-0371-86658258 +8613203830695 |
China | 29871 | 58 | ||
| +86 18953170293 | China | 2930 | 58 | ||
| +86-755-89396905 +86-15013857715 |
China | 10453 | 58 |